Oligonucleotide-based strategies to combat polyglutamine diseases
- PMID: 24848018
- PMCID: PMC4066792
- DOI: 10.1093/nar/gku385
Oligonucleotide-based strategies to combat polyglutamine diseases
Abstract
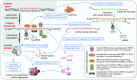
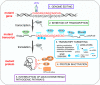
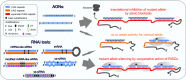
Considerable advances have been recently made in understanding the molecular aspects of pathogenesis and in developing therapeutic approaches for polyglutamine (polyQ) diseases. Studies on pathogenic mechanisms have extended our knowledge of mutant protein toxicity, confirmed the toxicity of mutant transcript and identified other toxic RNA and protein entities. One very promising therapeutic strategy is targeting the causative gene expression with oligonucleotide (ON) based tools. This straightforward approach aimed at halting the early steps in the cascade of pathogenic events has been widely tested for Huntington's disease and spinocerebellar ataxia type 3. In this review, we gather information on the use of antisense oligonucleotides and RNA interference triggers for the experimental treatment of polyQ diseases in cellular and animal models. We present studies testing non-allele-selective and allele-selective gene silencing strategies. The latter include targeting SNP variants associated with mutations or targeting the pathologically expanded CAG repeat directly. We compare gene silencing effectors of various types in a number of aspects, including their design, efficiency in cell culture experiments and pre-clinical testing. We discuss advantages, current limitations and perspectives of various ON-based strategies used to treat polyQ diseases.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Orr H.T., Zoghbi H.Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 2007;30:575–621. - PubMed
-
- Moseley M.L., Zu T., Ikeda Y., Gao W., Mosemiller A.K., Daughters R.S., Chen G., Weatherspoon M.R., Clark H.B., Ebner T.J., et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat. Genet. 2006;38:758–769. - PubMed
-
- Sharp A.H., Loev S.J., Schilling G., Li S.-H., Li X.-J., Bao J., Wagster M. V, Kotzuk J.A., Steiner J.P., Lo A., et al. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron. 1995;14:1065–1074. - PubMed
-
- Riley B.E., Orr H.T. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20:2183–2192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

