Brain pericytes acquire a microglial phenotype after stroke
- PMID: 24848101
- PMCID: PMC4131168
- DOI: 10.1007/s00401-014-1295-x
Brain pericytes acquire a microglial phenotype after stroke
Abstract
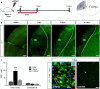
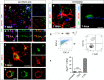
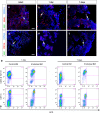
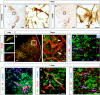
Pericytes are located on the abluminal side of endothelial cells lining the microvasculature in all organs. They have been identified as multipotent progenitor cells in several tissues of the body including the human brain. New evidence suggests that pericytes contribute to tissue repair, but their role in the injured brain is largely unknown. Here, we investigate the role of pericytes in ischemic stroke. Using a pericyte-reporter mouse model, we provide unique evidence that regulator of G-protein signaling 5 expressing cells are activated pericytes that leave the blood vessel wall, proliferate and give rise to microglial cells after ischemic brain injury. Consistently, we show that activated pericytes express microglial markers in human stroke brain tissue. We demonstrate that human brain-derived pericytes adopt a microglial phenotype and upregulate mRNA specific for activated microglial cells under hypoxic conditions in vitro. Our study indicates that the vasculature is a novel source of inflammatory cells with a microglial phenotype in brain ischemia and hence identifies pericytes as an important new target for the development of future stroke therapies.
Figures





References
-
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117(2):145–152. - PubMed
-
- Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, Sugimori H, Ooboshi H, Sasaki T, Kitazono T. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9(1):1–9. - PubMed
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. - PubMed
-
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52(2):127–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

