Cytoplasmic localization of p21 protects trophoblast giant cells from DNA damage induced apoptosis
- PMID: 24848107
- PMCID: PMC4029599
- DOI: 10.1371/journal.pone.0097434
Cytoplasmic localization of p21 protects trophoblast giant cells from DNA damage induced apoptosis
Abstract
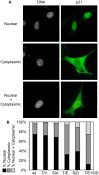
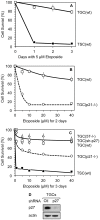
Proliferating trophoblast stem cells (TSCs) can differentiate into nonproliferating but viable trophoblast giant cells (TGCs) that are resistant to DNA damage induced apoptosis. Differentiation is associated with selective up-regulation of the Cip/Kip cyclin-dependent kinase inhibitors p57 and p21; expression of p27 remains constant. Previous studies showed that p57 localizes to the nucleus in TGCs where it is essential for endoreplication. Here we show that p27 also remains localized to the nucleus during TSC differentiation where it complements the role of p57. Unexpectedly, p21 localized to the cytoplasm where it was maintained throughout both the G- and S-phases of endocycles, and where it prevented DNA damage induced apoptosis. This unusual status for a Cip/Kip protein was dependent on site-specific phosphorylation of p21 by the Akt1 kinase that is also up-regulated in TGCs. Although cytoplasmic p21 is widespread among cancer cells, among normal cells it has been observed only in monocytes. The fact that it also occurs in TGCs reveals that p57 and p21 serve nonredundant functions, and suggests that the role of p21 in suppressing apoptosis is restricted to terminally differentiated cells.
Conflict of interest statement
Figures
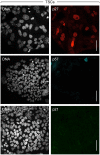

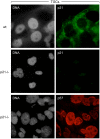
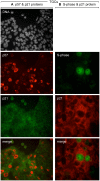
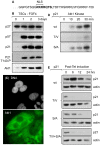


Similar articles
-
The dual roles of geminin during trophoblast proliferation and differentiation.Dev Biol. 2014 Mar 1;387(1):49-63. doi: 10.1016/j.ydbio.2013.12.034. Epub 2014 Jan 9. Dev Biol. 2014. PMID: 24412371 Free PMC article.
-
Checkpoint kinase 1 prevents cell cycle exit linked to terminal cell differentiation.Mol Cell Biol. 2011 Oct;31(19):4129-43. doi: 10.1128/MCB.05723-11. Epub 2011 Jul 26. Mol Cell Biol. 2011. PMID: 21791608 Free PMC article.
-
Development of mice without Cip/Kip CDK inhibitors.Biochem Biophys Res Commun. 2012 Oct 19;427(2):285-92. doi: 10.1016/j.bbrc.2012.09.041. Epub 2012 Sep 18. Biochem Biophys Res Commun. 2012. PMID: 23000166
-
Cellular Response upon Stress: p57 Contribution to the Final Outcome.Mediators Inflamm. 2015;2015:259325. doi: 10.1155/2015/259325. Epub 2015 Sep 27. Mediators Inflamm. 2015. PMID: 26491224 Free PMC article. Review.
-
Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors.Cell Cycle. 2010 Jun 15;9(12):2342-52. doi: 10.4161/cc.9.12.11988. Epub 2010 Jun 15. Cell Cycle. 2010. PMID: 20519948 Free PMC article. Review.
Cited by
-
GPER1 Signaling Initiates Migration of Female V-SVZ-Derived Cells.iScience. 2020 May 22;23(5):101077. doi: 10.1016/j.isci.2020.101077. Epub 2020 Apr 18. iScience. 2020. PMID: 32361597 Free PMC article.
-
[Statins enhance anti-tumor effect of suberoylanilide hydroxamic acid on human non-small cell lung carcinoma cells].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015 Sep;44(5):500-5. doi: 10.3785/j.issn.1008-9292.2015.09.05. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015. PMID: 26713523 Free PMC article. Chinese.
-
Links between DNA Replication, Stem Cells and Cancer.Genes (Basel). 2017 Jan 25;8(2):45. doi: 10.3390/genes8020045. Genes (Basel). 2017. PMID: 28125050 Free PMC article. Review.
-
IFITM1 suppression blocks proliferation and invasion of aromatase inhibitor-resistant breast cancer in vivo by JAK/STAT-mediated induction of p21.Cancer Lett. 2017 Jul 28;399:29-43. doi: 10.1016/j.canlet.2017.04.005. Epub 2017 Apr 12. Cancer Lett. 2017. PMID: 28411130 Free PMC article.
-
Resistance training suppresses accumulation of senescent fibro-adipogenic progenitors and senescence-associated secretory phenotype in aging rat skeletal muscle.Geroscience. 2025 Apr;47(2):1669-1683. doi: 10.1007/s11357-024-01338-2. Epub 2024 Sep 19. Geroscience. 2025. PMID: 39298108 Free PMC article.
References
-
- Dillon RL, White DE, Muller WJ (2007) The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26: 1338–1345. - PubMed
-
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, et al. (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3: 245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

