Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C
- PMID: 24848265
- PMCID: PMC4345833
- DOI: 10.1136/gutjnl-2013-306155
Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C
Abstract
Objective: Although direct-acting antiviral agents (DAAs) have markedly improved the outcome of treatment in chronic HCV infection, there continues to be an unmet medical need for improved therapies in difficult-to-treat patients as well as liver graft infection. Viral entry is a promising target for antiviral therapy.
Design: Aiming to explore the role of entry inhibitors for future clinical development, we investigated the antiviral efficacy and toxicity of entry inhibitors in combination with DAAs or other host-targeting agents (HTAs). Screening a large series of combinations of entry inhibitors with DAAs or other HTAs, we uncovered novel combinations of antivirals for prevention and treatment of HCV infection.
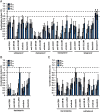
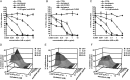
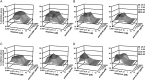
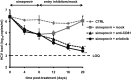
Results: Combinations of DAAs or HTAs and entry inhibitors including CD81-, scavenger receptor class B type I (SR-BI)- or claudin-1 (CLDN1)-specific antibodies or small-molecule inhibitors erlotinib and dasatinib were characterised by a marked and synergistic inhibition of HCV infection over a broad range of concentrations with undetectable toxicity in experimental designs for prevention and treatment both in cell culture models and in human liver-chimeric uPA/SCID mice.
Conclusions: Our results provide a rationale for the development of antiviral strategies combining entry inhibitors with DAAs or HTAs by taking advantage of synergy. The uncovered combinations provide perspectives for efficient strategies to prevent liver graft infection and novel interferon-free regimens.
Keywords: HCV; HEPATITIS C; LIVER.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures




Comment in
-
Viral entry inhibition: too late for hepatitis C, but promising for other viral infections.Gut. 2015 Mar;64(3):362-4. doi: 10.1136/gutjnl-2014-307452. Epub 2014 Aug 6. Gut. 2015. PMID: 25098973 No abstract available.
-
Inhibition of hepatitis C entry: too soon to dismiss while many are still being denied treatment.Gut. 2015 Apr;64(4):690-1. doi: 10.1136/gutjnl-2014-308396. Epub 2014 Oct 6. Gut. 2015. PMID: 25287483 No abstract available.
Similar articles
-
In vivo combination of human anti-envelope glycoprotein E2 and -Claudin-1 monoclonal antibodies for prevention of hepatitis C virus infection.Antiviral Res. 2019 Feb;162:136-141. doi: 10.1016/j.antiviral.2018.12.018. Epub 2018 Dec 30. Antiviral Res. 2019. PMID: 30599173 Free PMC article.
-
Humanisation of a claudin-1-specific monoclonal antibody for clinical prevention and cure of HCV infection without escape.Gut. 2018 Apr;67(4):736-745. doi: 10.1136/gutjnl-2016-312577. Epub 2017 Mar 30. Gut. 2018. PMID: 28360099 Free PMC article.
-
Entry inhibitors: New advances in HCV treatment.Emerg Microbes Infect. 2016 Jan 6;5(1):e3. doi: 10.1038/emi.2016.3. Emerg Microbes Infect. 2016. PMID: 26733381 Free PMC article. Review.
-
Entry inhibitors and future treatment of hepatitis C.Antiviral Res. 2014 Apr;104:136-42. doi: 10.1016/j.antiviral.2014.02.001. Epub 2014 Feb 10. Antiviral Res. 2014. PMID: 24525381 Review.
-
Monoclonal antibodies against extracellular domains of claudin-1 block hepatitis C virus infection in a mouse model.J Virol. 2015 May;89(9):4866-79. doi: 10.1128/JVI.03676-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673725 Free PMC article.
Cited by
-
Ritonavir Blocks Hepatitis E Virus Internalization and Clears Hepatitis E Virus In Vitro with Ribavirin.Viruses. 2022 Nov 3;14(11):2440. doi: 10.3390/v14112440. Viruses. 2022. PMID: 36366538 Free PMC article.
-
Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action.J Virol. 2015 Oct;89(19):10053-63. doi: 10.1128/JVI.01473-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202241 Free PMC article.
-
Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges.Gastroenterology. 2019 Jan;156(2):431-445. doi: 10.1053/j.gastro.2018.10.024. Epub 2018 Oct 17. Gastroenterology. 2019. PMID: 30342035 Free PMC article. Review.
-
Src Tyrosine Kinase Inhibitors: New Perspectives on Their Immune, Antiviral, and Senotherapeutic Potential.Front Pharmacol. 2019 Sep 18;10:1011. doi: 10.3389/fphar.2019.01011. eCollection 2019. Front Pharmacol. 2019. PMID: 31619990 Free PMC article. Review.
-
Disruption of Claudin-1 Expression by miRNA-182 Alters the Susceptibility to Viral Infectivity in HCV Cell Models.Front Genet. 2018 Mar 20;9:93. doi: 10.3389/fgene.2018.00093. eCollection 2018. Front Genet. 2018. PMID: 29616082 Free PMC article.
References
-
- Dabbouseh NM, Jensen DM. Future therapies for chronic hepatitis C. Nat Rev Gastroenterol Hepatol 2013;10:268–76. - PubMed
-
- Sarrazin C, Hezode C, Zeuzem S, et al. Antiviral strategies in hepatitis C virus infection. J Hepatol 2012;56(Suppl 1):S88–100. - PubMed
-
- Chung RT, Baumert TF. Curing chronic hepatitis C—the arc of a medical triumph. N Engl J Med 2014;370:1576–8. - PubMed
-
- Crespo G, Marino Z, Navasa M, et al. Viral hepatitis in liver transplantation. Gastroenterology 2012;142:1373–83 e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
