Overexpression of a defensin enhances resistance to a fruit-specific anthracnose fungus in pepper
- PMID: 24848280
- PMCID: PMC4029827
- DOI: 10.1371/journal.pone.0097936
Overexpression of a defensin enhances resistance to a fruit-specific anthracnose fungus in pepper
Abstract
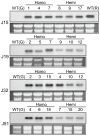
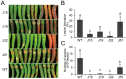
Functional characterization of a defensin, J1-1, was conducted to evaluate its biotechnological potentiality in transgenic pepper plants against the causal agent of anthracnose disease, Colletotrichum gloeosporioides. To determine antifungal activity, J1-1 recombinant protein was generated and tested for the activity against C. gloeosporioides, resulting in 50% inhibition of fungal growth at a protein concentration of 0.1 mg·mL-1. To develop transgenic pepper plants resistant to anthracnose disease, J1-1 cDNA under the control of 35S promoter was introduced into pepper via Agrobacterium-mediated genetic transformation method. Southern and Northern blot analyses confirmed that a single copy of the transgene in selected transgenic plants was normally expressed and also stably transmitted to subsequent generations. The insertion of T-DNA was further analyzed in three independent homozygous lines using inverse PCR, and confirmed the integration of transgene in non-coding region of genomic DNA. Immunoblot results showed that the level of J1-1 proteins, which was not normally accumulated in unripe fruits, accumulated high in transgenic plants but appeared to differ among transgenic lines. Moreover, the expression of jasmonic acid-biosynthetic genes and pathogenesis-related genes were up-regulated in the transgenic lines, which is co-related with the resistance of J1-1 transgenic plants to anthracnose disease. Consequently, the constitutive expression of J1-1 in transgenic pepper plants provided strong resistance to the anthracnose fungus that was associated with highly reduced lesion formation and fungal colonization. These results implied the significance of the antifungal protein, J1-1, as a useful agronomic trait to control fungal disease.
Conflict of interest statement
Figures






Similar articles
-
Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants.Planta. 2016 Aug;244(2):379-92. doi: 10.1007/s00425-016-2514-6. Epub 2016 Apr 13. Planta. 2016. PMID: 27074836
-
A Colletotrichum gloeosporioides-induced esterase gene of nonclimacteric pepper (Capsicum annuum) fruit during ripening plays a role in resistance against fungal infection.Plant Mol Biol. 2005 Jul;58(4):529-41. doi: 10.1007/s11103-005-7088-9. Plant Mol Biol. 2005. PMID: 16021337
-
Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato.Plant Mol Biol. 2020 Nov;104(4-5):381-395. doi: 10.1007/s11103-020-01046-3. Epub 2020 Aug 16. Plant Mol Biol. 2020. PMID: 32803478
-
Breeding for Anthracnose Disease Resistance in Chili: Progress and Prospects.Int J Mol Sci. 2018 Oct 11;19(10):3122. doi: 10.3390/ijms19103122. Int J Mol Sci. 2018. PMID: 30314374 Free PMC article. Review.
-
Molecular and cellular control of cell death and defense signaling in pepper.Planta. 2015 Jan;241(1):1-27. doi: 10.1007/s00425-014-2171-6. Epub 2014 Sep 25. Planta. 2015. PMID: 25252816 Review.
Cited by
-
Transgenic and genome-edited fruits: background, constraints, benefits, and commercial opportunities.Hortic Res. 2021 Jul 17;8(1):166. doi: 10.1038/s41438-021-00601-3. Hortic Res. 2021. PMID: 34274949 Free PMC article. Review.
-
Structural Diversity and Highly Specific Host-Pathogen Transcriptional Regulation of Defensin Genes Is Revealed in Tomato.Int J Mol Sci. 2020 Dec 9;21(24):9380. doi: 10.3390/ijms21249380. Int J Mol Sci. 2020. PMID: 33317090 Free PMC article.
-
Antimicrobial Peptides from Fruits and Their Potential Use as Biotechnological Tools-A Review and Outlook.Front Microbiol. 2017 Jan 10;7:2136. doi: 10.3389/fmicb.2016.02136. eCollection 2016. Front Microbiol. 2017. PMID: 28119671 Free PMC article. Review.
-
Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake.Plant Biotechnol J. 2021 Sep;19(9):1756-1768. doi: 10.1111/pbi.13589. Epub 2021 May 4. Plant Biotechnol J. 2021. PMID: 33774895 Free PMC article.
-
Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants.Planta. 2016 Aug;244(2):379-92. doi: 10.1007/s00425-016-2514-6. Epub 2016 Apr 13. Planta. 2016. PMID: 27074836
References
-
- Dixon RA (2001) Natural products and plant disease resistance. Nature 411: 843–847. - PubMed
-
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329. - PubMed
-
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227. - PubMed
-
- Fritig B, Heitz T, Legrand M (1998) Antimicrobial proteins in induced plant defense. Curr Opin Immunol 10: 16–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

