Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala
- PMID: 24848467
- PMCID: PMC4122738
- DOI: 10.1152/jn.00597.2013
Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala
Abstract
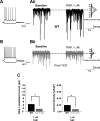
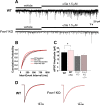
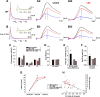
Fragile X syndrome (FXS) is the leading cause of inherited intellectual disability. Comorbidities of FXS such as autism are increasingly linked to imbalances in excitation and inhibition (E/I) as well as dysfunction in GABAergic transmission in a number of brain regions including the amygdala. However, the link between E/I imbalance and GABAergic transmission deficits in the FXS amygdala is poorly understood. Here we reveal that normal tonic GABAA receptor-mediated neurotransmission in principal neurons (PNs) of the basolateral amygdala (BLA) is comprised of both δ- and α5-subunit-containing GABAA receptors. Furthermore, tonic GABAergic capacity is reduced in these neurons in the Fmr1 knockout (KO) mouse model of FXS (1.5-fold total, 3-fold δ-subunit, and 2-fold α5-subunit mediated) as indicated by application of gabazine (50 μM), 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, 1 μM), and α5ia (1.5 μM) in whole cell patch-clamp recordings. Moreover, α5-containing tonic GABAA receptors appear to preferentially modulate nonsomatic compartments of BLA PNs. Examination of evoked feedforward synaptic transmission in these cells surprisingly revealed no differences in overall synaptic conductance or E/I balance between wild-type (WT) and Fmr1 KO mice. Instead, we observed altered feedforward kinetics in Fmr1 KO PNs that supports a subtle yet significant decrease in E/I balance at the peak of excitatory conductance. Blockade of α5-subunit-containing GABAA receptors replicated this condition in WT PNs. Therefore, our data suggest that tonic GABAA receptor-mediated neurotransmission can modulate synaptic E/I balance and timing established by feedforward inhibition and thus may represent a therapeutic target to enhance amygdala function in FXS.
Keywords: GABA; amygdala; fragile X syndrome; tonic inhibition.
Copyright © 2014 the American Physiological Society.
Figures


Similar articles
-
Rescue of deficient amygdala tonic γ-aminobutyric acidergic currents in the Fmr-/y mouse model of fragile X syndrome by a novel γ-aminobutyric acid type A receptor-positive allosteric modulator.J Neurosci Res. 2016 Jun;94(6):568-78. doi: 10.1002/jnr.23632. Epub 2015 Aug 26. J Neurosci Res. 2016. PMID: 26308557 Free PMC article.
-
Decreased surface expression of the δ subunit of the GABAA receptor contributes to reduced tonic inhibition in dentate granule cells in a mouse model of fragile X syndrome.Exp Neurol. 2017 Nov;297:168-178. doi: 10.1016/j.expneurol.2017.08.008. Epub 2017 Aug 16. Exp Neurol. 2017. PMID: 28822839 Free PMC article.
-
Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome.J Neurosci. 2010 Jul 21;30(29):9929-38. doi: 10.1523/JNEUROSCI.1714-10.2010. J Neurosci. 2010. PMID: 20660275 Free PMC article.
-
Fragile X syndrome: the GABAergic system and circuit dysfunction.Dev Neurosci. 2011;33(5):349-64. doi: 10.1159/000329420. Epub 2011 Sep 21. Dev Neurosci. 2011. PMID: 21934270 Free PMC article. Review.
-
Impaired GABA Neural Circuits Are Critical for Fragile X Syndrome.Neural Plast. 2018 Oct 3;2018:8423420. doi: 10.1155/2018/8423420. eCollection 2018. Neural Plast. 2018. PMID: 30402088 Free PMC article. Review.
Cited by
-
Altered nocifensive behavior in animal models of autism spectrum disorder: The role of the nicotinic cholinergic system.Neuropharmacology. 2016 Dec;111:323-334. doi: 10.1016/j.neuropharm.2016.09.013. Epub 2016 Sep 13. Neuropharmacology. 2016. PMID: 27638450 Free PMC article.
-
Compromising the phosphodependent regulation of the GABAAR β3 subunit reproduces the core phenotypes of autism spectrum disorders.Proc Natl Acad Sci U S A. 2015 Dec 1;112(48):14805-10. doi: 10.1073/pnas.1514657112. Epub 2015 Nov 16. Proc Natl Acad Sci U S A. 2015. PMID: 26627235 Free PMC article.
-
Olfactory dysfunction and altered cortical excitability in the mouse model of Fragile X Syndrome.Biol Res. 2025 Apr 24;58(1):21. doi: 10.1186/s40659-024-00582-2. Biol Res. 2025. PMID: 40275427 Free PMC article.
-
Control of Behavioral Arousal and Defense by a Glutamatergic Midbrain-Amygdala Pathway in Mice.Front Neurosci. 2022 Apr 18;16:850193. doi: 10.3389/fnins.2022.850193. eCollection 2022. Front Neurosci. 2022. PMID: 35527820 Free PMC article.
-
Rett Syndrome and Fragile X Syndrome: Different Etiology With Common Molecular Dysfunctions.Front Cell Neurosci. 2021 Nov 19;15:764761. doi: 10.3389/fncel.2021.764761. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34867203 Free PMC article. Review.
References
-
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59: 167–171, 2010 - PubMed
-
- Ali AB, Thomson AM. Synaptic alpha5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex 18: 1260–1271, 2008 - PubMed
-
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther 125: 11–26, 2010 - PubMed
-
- Atack JR, Pike A, Clarke A, Cook SM, Sohal B, McKernan RM, Dawson GR. Rat pharmacokinetics and pharmacodynamics of a sustained release formulation of the GABAA alpha5-selective compound L-655,708. Drug Metab Dispos 34: 887–893, 2006 - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370–377, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

