Delivery of antibodies to the cytosol: debunking the myths
- PMID: 24848507
- PMCID: PMC4171028
- DOI: 10.4161/mabs.29268
Delivery of antibodies to the cytosol: debunking the myths
Abstract
The use of antibodies to target their antigens in living cells is a powerful analytical tool for cell biology research. Not only can molecules be localized and visualized in living cells, but interference with cellular processes by antibodies may allow functional analysis down to the level of individual post-translational modifications and splice variants, which is not possible with genetic or RNA-based methods. To utilize the vast resource of available antibodies, an efficient system to deliver them into the cytosol from the outside is needed. Numerous strategies have been proposed, but the most robust and widely applicable procedure still remains to be identified, since a quantitative ranking of the efficiencies has not yet been done. To achieve this, we developed a novel efficiency evaluation method for antibody delivery based on a fusion protein consisting of a human IgG 1 Fc and the recombination enzyme Cre (Fc-Cre). Applied to suitable GFP reporter cells, it allows the important distinction between proteins trapped in endosomes and those delivered to the cytosol. Further, it ensures viability of positive cells and is unsusceptible to fixation artifacts and misinterpretation of cellular localization in microscopy and flow cytometry. Very low cytoplasmic delivery efficiencies were found for various profection reagents and membrane penetrating peptides, leaving electroporation as the only practically useful delivery method for antibodies. This was further verified by the successful application of this method to bind antibodies to cytosolic components in living cells.
Keywords: CPP; PTD; cell penetrating peptides; electroporation; electrotransfer; profection; protein delivery; protein transduction domain; transbody; transfection; yumab.
Figures





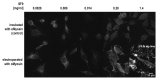
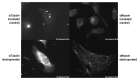
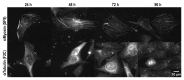
Similar articles
-
Evaluating the Delivery of Proteins to the Cytosol of Mammalian Cells.Methods Mol Biol. 2017;1513:201-208. doi: 10.1007/978-1-4939-6539-7_14. Methods Mol Biol. 2017. PMID: 27807839
-
Quantitative assessment of cellular uptake and cytosolic access of antibody in living cells by an enhanced split GFP complementation assay.Biochem Biophys Res Commun. 2015 Nov 27;467(4):771-7. doi: 10.1016/j.bbrc.2015.10.066. Epub 2015 Oct 19. Biochem Biophys Res Commun. 2015. PMID: 26482850
-
Engineering of a tumor cell-specific, cytosol-penetrating antibody with high endosomal escape efficacy.Biochem Biophys Res Commun. 2018 Sep 18;503(4):2510-2516. doi: 10.1016/j.bbrc.2018.07.008. Epub 2018 Jul 4. Biochem Biophys Res Commun. 2018. PMID: 30208519
-
Finding ways into the cytosol: Peptide-mediated approaches for delivering proteins into cells.Curr Opin Chem Biol. 2024 Aug;81:102482. doi: 10.1016/j.cbpa.2024.102482. Epub 2024 Jun 20. Curr Opin Chem Biol. 2024. PMID: 38905721 Review.
-
The taming of the cell penetrating domain of the HIV Tat: myths and realities.J Control Release. 2007 Feb 12;117(2):148-62. doi: 10.1016/j.jconrel.2006.10.031. Epub 2006 Nov 17. J Control Release. 2007. PMID: 17196289 Free PMC article. Review.
Cited by
-
Imaging of native transcription factors and histone phosphorylation at high resolution in live cells.J Cell Biol. 2018 Apr 2;217(4):1537-1552. doi: 10.1083/jcb.201709153. Epub 2018 Feb 12. J Cell Biol. 2018. PMID: 29440513 Free PMC article.
-
Intracellular delivery of therapeutic proteins. New advancements and future directions.Front Bioeng Biotechnol. 2023 May 25;11:1211798. doi: 10.3389/fbioe.2023.1211798. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37304137 Free PMC article. Review.
-
The penetration of a charged peptide across a membrane under an external electric field: a coarse-grained molecular dynamics simulation.RSC Adv. 2018 Dec 11;8(72):41517-41525. doi: 10.1039/c8ra07654e. eCollection 2018 Dec 7. RSC Adv. 2018. PMID: 35559300 Free PMC article.
-
Targeting the Inside of Cells with Biologicals: Toxin Routes in a Therapeutic Context.BioDrugs. 2023 Mar;37(2):181-203. doi: 10.1007/s40259-023-00580-y. Epub 2023 Feb 2. BioDrugs. 2023. PMID: 36729328 Free PMC article. Review.
-
Specific in vivo knockdown of protein function by intrabodies.MAbs. 2015;7(6):1010-35. doi: 10.1080/19420862.2015.1076601. Epub 2015 Aug 7. MAbs. 2015. PMID: 26252565 Free PMC article. Review.
References
-
- Gawlitta W, Osborn M, Weber K. Coiling of intermediate filaments induced by microinjection of a vimentin-specific antibody does not interfere with locomotion and mitosis. Eur J Cell Biol. 1981;26:83–90. - PubMed
-
- Kallajoki M, Harborth J, Weber K, Osborn M. Microinjection of a monoclonal antibody against SPN antigen, now identified by peptide sequences as the NuMA protein, induces micronuclei in PtK2 cells. J Cell Sci. 1993;104:139–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
