β-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma
- PMID: 24848510
- PMCID: PMC4134699
- DOI: 10.1158/0008-5472.CAN-13-3275
β-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma
Abstract
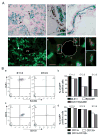
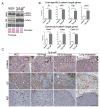
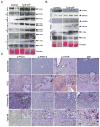
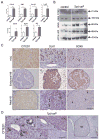
Hepatocellular carcinoma (HCC) was thought historically to arise from hepatocytes, but gene expression studies have suggested that it can also arise from fetal progenitor cells or their adult progenitor progeny. Here, we report the identification of a unique population of fetal liver progenitor cells in mice that can serve as a cell of origin in HCC development. In the transgenic model used, mice carry the Cited1-CreER(TM)-GFP BAC transgene in which a tamoxifen-inducible Cre (CreER(TM)) and GFP are controlled by a 190-kb 5' genomic region of Cited1, a transcriptional coactivator protein for CBP/p300. Wnt signaling is critical for regulating self-renewal of progenitor/stem cells and has been implicated in the etiology of cancers of rapidly self-renewing tissues, so we hypothesized that Wnt pathway activation in CreER(TM)-GFP(+) progenitors would result in HCC. In livers from the mouse model, transgene-expressing cells represented 4% of liver cells at E11.5 when other markers were expressed, characteristic of the hepatic stem/progenitor cells that give rise to adult hepatocytes, cholangiocytes, and SOX9(+) periductal cells. By 26 weeks of age, more than 90% of Cited1-CreER(TM)-GFP;Ctnnb1(ex3(fl)) mice with Wnt pathway activation developed HCC and, in some cases, hepatoblastomas and lung metastases. HCC and hepatoblastomas resembled their human counterparts histologically, showing activation of Wnt, Ras/Raf/MAPK, and PI3K/AKT/mTOR pathways and expressing relevant stem/progenitor cell markers. Our results show that Wnt pathway activation is sufficient for malignant transformation of these unique liver progenitor cells, offering functional support for a fetal/adult progenitor origin of some human HCC. We believe this model may offer a valuable new tool to improve understanding of the cellular etiology and biology of HCC and hepatoblastomas and the development of improved therapeutics for these diseases.
©2014 American Association for Cancer Research.
Conflict of interest statement
No conflicts to disclose.
Figures

References
-
- El-Serag HB. CURRENT CONCEPTS Hepatocellular Carcinoma. New England Journal of Medicine. 2011;365:1118–27. - PubMed
-
- Thorgeirsson SS, Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature Medicine. 2006;12:410–6. - PubMed
-
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. - PubMed
-
- Guichard C, Imbeaud S, Amaddeo G, Ben Maad I, Letouze E, Pelletier L, et al. Landscape of Somatic Mutation in Hepatocellular Carcinoma. Journal of Hepatology. 2012;56:S8-S.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

