IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells
- PMID: 24848710
- PMCID: PMC4121023
- DOI: 10.1210/jc.2014-1139
IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells
Abstract
Context: FSH is routinely administered to in vitro fertilization patients to induce follicle maturation. During this process, granulosa cells differentiate and acquire specific functional characteristics that are required to coordinate ovulation and oocyte maturation.
Objective: The objective of the study was to gain insight into the molecular mechanisms regulating human granulosa cell differentiation. Design, Setting, Patients, and Interventions: Cumulus and mural granulosa cells were isolated from the follicular aspirates of in vitro fertilization patients and analyzed immediately or cultured in serum-free media in the presence of FSH, IGFs, or an inhibitor of type I IGF receptor (IGF1R) activity.
Main outcome: We quantified the mRNA and protein levels of steroidogenic enzymes, components of the IGF system, and gonadotropin receptors; measured 17β-estradiol levels; and examined the activation of intracellular signaling pathways to assess the granulosa cell differentiation as well as the FSH and IGF actions in both cumulus and mural cells.
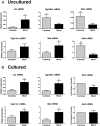
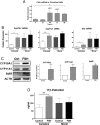
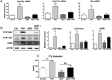
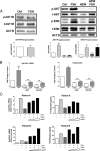
Results: In freshly isolated cells, LH receptor (Lhr) and steroidogenic acute regulator (Star) were expressed at lower levels in cumulus than mural cells, whereas FSH receptor (Fshr) and anti-Müllerian hormone (Amh) were expressed at higher levels in cumulus than mural cells. In vitro, the expression of Igf2, the differentiation markers Lhr, Star, and Cyp19a1 (aromatase) as well as 17β-estradiol production remained low in untreated cumulus cells but increased significantly after FSH treatment. Strikingly, this stimulatory effect of FSH was abolished by the inhibition of IGF1R activity. FSH-induced activation of v-akt murine thymoma viral oncogene homolog 3 (AKT) required IGF1R activity, and overexpression of constitutively active AKT rescued the induction of differentiation markers and 17β-estradiol production by FSH in the presence of the IGF1R inhibitor.
Conclusions: The cumulus cell response to FSH resembles the differentiation of preantral to preovulatory granulosa cells. This differentiation program requires IGF1R activity and subsequent AKT activation.
Figures

References
-
- Li RH, Ng EH. Management of anovulatory infertility. Best practice, research. Clin Obstet Gynaecol. 2012;26:757–768 - PubMed
-
- Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2011 assisted reproductive technology fertility clinic success rates report. Atlanta (GA): US Department of Health and Human Services; 2013
-
- Sunderam S, Kissin DM, Flowers L, et al. Assisted reproductive technology surveillance—United States, 2009. MMWR Surveill Summ. 2012;61:1–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

