Oral herbal therapies for treating osteoarthritis
- PMID: 24848732
- PMCID: PMC4494689
- DOI: 10.1002/14651858.CD002947.pub2
Oral herbal therapies for treating osteoarthritis
Abstract
Background: Medicinal plant products are used orally for treating osteoarthritis. Although their mechanisms of action have not yet been elucidated in full detail, interactions with common inflammatory mediators provide a rationale for using them to treat osteoarthritic complaints.
Objectives: To update a previous Cochrane review to assess the benefits and harms of oral medicinal plant products in treating osteoarthritis.
Search methods: We searched electronic databases (CENTRAL, MEDLINE, EMBASE, AMED, CINAHL, ISI Web of Science, World Health Organization Clinical Trials Registry Platform) to 29 August 2013, unrestricted by language, and the reference lists from retrieved trials.
Selection criteria: Randomised controlled trials of orally consumed herbal interventions compared with placebo or active controls in people with osteoarthritis were included. Herbal interventions included any plant preparation but excluded homeopathy or aromatherapy products, or any preparation of synthetic origin.
Data collection and analysis: Two authors used standard methods for trial selection and data extraction, and assessed the quality of the body of evidence using the GRADE approach for major outcomes (pain, function, radiographic joint changes, quality of life, withdrawals due to adverse events, total adverse events, and serious adverse events).
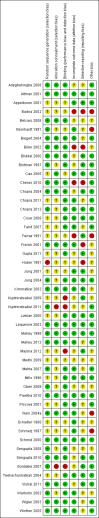
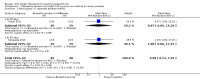
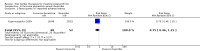
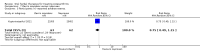
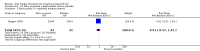
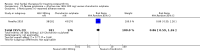
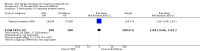
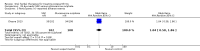
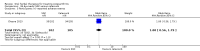
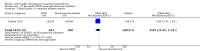
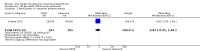
Main results: Forty-nine randomised controlled studies (33 interventions, 5980 participants) were included. Seventeen studies of confirmatory design (sample and effect sizes pre-specified) were mostly at moderate risk of bias. The remaining 32 studies of exploratory design were at higher risk of bias. Due to differing interventions, meta-analyses were restricted to Boswellia serrata (monoherbal) and avocado-soyabean unsaponifiables (ASU) (two herb combination) products.Five studies of three different extracts from Boswellia serrata were included. High-quality evidence from two studies (85 participants) indicated that 90 days treatment with 100 mg of enriched Boswellia serrata extract improved symptoms compared to placebo. Mean pain was 40 points on a 0 to 100 point VAS scale (0 is no pain) with placebo, enriched Boswellia serrata reduced pain by a mean of 17 points (95% confidence interval (CI) 8 to 26); number needed to treat for an additional beneficial outcome (NNTB) 2; the 95% CIs did not exclude a clinically significant reduction of 15 points in pain. Physical function was 33 points on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) 0 to 100 point subscale (0 is no loss of function) with placebo, enriched Boswellia serrata improved function by 8 points (95% CI 2 to 14); NNTB 4. Assuming a minimal clinically important difference of 10 points, we cannot exclude a clinically important benefit in some people. Moderate-quality evidence (one study, 96 participants) indicated that adverse events were probably reduced with enriched Boswellia serrata (18/48 events versus 30/48 events with placebo; relative risk (RR) 0.60, 95% CI 0.39 to 0.92). Possible benefits of other Boswellia serrata extracts over placebo were confirmed in moderate-quality evidence from two studies (97 participants) of Boswellia serrata (enriched) 100 mg plus non-volatile oil, and low-quality evidence from small single studies of a 999 mg daily dose of Boswellia serrata extract and 250 mg daily dose of enrichedBoswellia serrata. It was uncertain if a 99 mg daily dose of Boswellia serrata offered benefits over valdecoxib due to the very low-quality evidence from a small single study. It was uncertain if there was an increased risk of adverse events or withdrawals with Boswellia serrata extract due to variable reporting of results across studies. The studies reported no serious adverse events. Quality of life and radiographic joint changes were not measured.Six studies examined the ASU product Piasclidine®. Moderate-quality evidence from four studies (651 participants) indicated that ASU 300 mg produced a small and clinically questionable improvement in symptoms, and probably no increased adverse events compared to placebo after three to 12 months treatment. Mean pain with placebo was 40.5 points on a VAS 0 to 100 scale (0 is no pain), ASU 300 mg reduced pain by a mean of 8.5 points (95% CI 1 to 16 points); NNTB 8. ASU 300 mg improved function (standardised mean difference (SMD) -0.42, 95% CI -0.73 to -0.11). Function was estimated as 47 mm (0 to 100 mm scale, where 0 is no loss of function) with placebo, ASU 300 mg improved function by a mean of 7 mm (95% CI 2 to 12 mm); NNTB 5 (3 to 19). There were no differences in adverse events (5 studies, 1050 participants) between ASU (53%) and placebo (51%) (RR 1.04, 95% CI 0.97 to 1.12); withdrawals due to adverse events (1 study, 398 participants) between ASU (17%) and placebo (15%) (RR 1.14, 95% CI 0.73 to 1.80); or serious adverse events (1 study, 398 participants) between ASU (40%) and placebo (33%) (RR 1.22, 95% CI 0.94 to 1.59). Radiographic joint changes, measured as change in joint space width (JSW) in two studies (453 participants) did not differ between ASU 300 mg treatment (-0.53 mm) and placebo (-0.65 mm); mean difference of -0.12 (95% CI -0.43 to 0.19). Moderate-quality evidence from a single study (156 participants) confirmed possible benefits of ASU 600 mg over placebo, with no increased adverse events. Low-quality evidence (1 study, 357 participants) indicated there may be no differences in symptoms or adverse events between ASU 300 mg and chondroitin sulphate. Quality of life was not measured.All other herbal interventions were investigated in single studies, limiting conclusions. No serious side effects related to any plant product were reported.
Authors' conclusions: Evidence for the proprietary ASU product Piasclidine® in the treatment of osteoarthritis symptoms seems moderate to high for short term use, but studies over a longer term and against an apparently active control are less convincing. Several other medicinal plant products, including extracts of Boswellia serrata, show trends of benefits that warrant further investigation in light of the fact that the risk of adverse events appear low.There is no evidence that Piasclidine® significantly improves joint structure, and limited evidence that it prevents joint space narrowing. Structural changes were not tested for with any other herbal intervention.Further investigations are required to determine optimum daily doses producing clinical benefits without adverse events.
Conflict of interest statement
None known
Figures
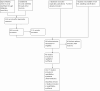






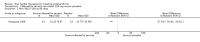

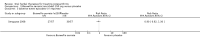




























































































Update of
-
Herbal therapy for treating osteoarthritis.Cochrane Database Syst Rev. 2001;(1):CD002947. doi: 10.1002/14651858.CD002947. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD002947. doi: 10.1002/14651858.CD002947.pub2. PMID: 11279783 Updated.
References
References to studies included in this review
Adegbehingbe 2008 {published data only}
Altman 2001 {published data only}
-
- Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis & Rheumatism 2001;44(11):2531‐8. - PubMed
Appelboom 2001 {published data only}
-
- Appelboom T, Schuermans J, Verbruggen G, Henrotin Y, Reginster JY. Symptoms modifying effect of avocado/soyabean unsaponifiables (ASU) in knee osteoarthritis: A double‐blind, prospective, placebo‐controlled study. Scandinavian Journal of Rheumatology 2001;30(4):242‐7. - PubMed
Badria 2002 {published data only}
-
- Badria FA, El‐Farahaty T, Shabana AA, Hawas SA, El‐Batoty MF. Boswellia‐curcumin preparation for treating knee osteoarthritis: A clinical evaluation. Alternative & Complementary Therapies 2002;8(6):341‐8.
Belcaro 2008 {published data only}
-
- Belcaro G, Cesarone MR, Errichi S, Zulli C, Errichi BM, Vinciguerra G, et al. Treatment of osteoarthritis with Pycnogenol®. The SVOS (San Valentino Osteoarthritis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytotherapy Research 2008;22:518‐23. [DOI: 10.1002/ptr.2376] - DOI - PubMed
Bernhardt 1991 {unpublished data only}
-
- Bernhardt M, Keimel A, Belucci G, et al. Double‐blind, randomised, comparative study of Phytodolor N and placebo as well as an open comparison with Felden 20 Tabs involving convalescent inpatients with arthrotic joint alterations. Unpublished Pharma Report 1991. [Steigrwald Pharmaceuticals, Germany]
Biegert 2004 {published data only}
-
- Biegert C, Wagner I, Ludtke R, Kotter I, Lohmuller C, Gunaydin I, et al. Efficacy and safety of willow bark extract in the treatment of osteoarthritis and rheumatoid arthritis: Results of 2 randomized double‐blind controlled trials. The Journal of Rheumatology 2004;31(11):2121‐30. - PubMed
Biller 2002 {published data only}
-
- Biller A. Results of two randomized controlled studies and of a post‐marketing surveillance study investigating a Devil's claw extract [Ergebnisse zweier randomisierter kontrollierter Studien und einer Anwendungsbeobachtung mit Teufelskrallenextrakt]. In: Schulz V, Rietbrock N, Roots I, Loew D editor(s). In: Phylopharmaka VII. Darmstadt: Steinkopf‐Verlag, 2002:81‐92.
Bliddal 2000 {published data only}
-
- Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, et al. A randomized, placebo‐controlled, cross‐over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis and Cartilage 2000;8:9‐12. - PubMed
Blotman 1997 {published data only}
-
- Blotman F, Maheu E, Wulwik A, Caspard H, Lopez A. Efficacy and safety of avocado/soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip [Efficacite et tolerance des insaponifiables d'avocat/soja dans le traitement de la gonarthrose et de la coxarthrose symptomatiques]. Revue du Rhumatisme [Éd Fr Joint Bone Spine] 1997;64(12):825‐34. - PubMed
Cao 2005 {published data only}
Cheras 2010 {published data only}
Chopra 2004 {published data only}
-
- Chopra A, Lavin P, Patwardhan B, Chitre D. A 32‐week randomized, placebo‐controlled clinical evaluation of RA‐11, an Ayurvedic drug, on osteoarthritis of the knees. Journal of Clinical Rheumatology 2004;10(5):236‐45. - PubMed
Chopra 2011 {published data only}
-
- Chopra A, Saluja M, Tillu G, Venugopalan A, Sarmukaddam S, Raut AK, et al. A randomized controlled exploratory evaluation of standardized Ayurvedic formulations in symptomatic osteoarthritis knees: A Government of India NMITLI project. Evidence‐based Complementary and Alternative Medicine 2011;8:1‐12. [DOI: 10.1155/2011/724291] - DOI - PMC - PubMed
Chopra 2013 {published data only}
-
- Chopra A, Saluja M, Tillu G, Sarmukkaddam S, Venugopalan A, Narsimulu G, Handa R, et al. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double‐blind, controlled equivalence trial. Rheumatology 2013;52(8):1408‐17. [DOI: 10.1093/rheumatology/kes414] - DOI - PubMed
Cisar 2008 {published data only}
Farid 2007 {published data only}
Ferraz 1991 {published data only}
-
- Ferraz MB, Pereira RB, Iwata NM, Atra E. Tipi. A popular analgesic tea: A double‐blind cross‐over trial in osteoarthritis (letter). Clinical and Experimental Rheumatology 1991;9:205‐12. - PubMed
Frerick 2001 {published data only}
-
- Frerick H, Biller A, Schmidt U. A treatment schedule for coxarthrosis: A double‐blind study with Devil's claw [Stufenschema bei coxarthrose: Doppelblindstudie mit Teufelskralle]. Der Kassenarzt 2001;5:34‐41.
Gupta 2011 {published data only}
Huber 1991 {published data only (unpublished sought but not used)}
-
- Huber B. Therapy of degenerative rheumatic diseases: Use of analgesic medication with Phytodolor N [Therapie degenerativer rheumatischer erkrankungen: Bedarf an zusatzlicher analgetischer medikation unter Phytodolor N]. Fortschritte der Medizin 1991;109:248‐50. - PubMed
Jung 2001 {published data only}
-
- Jung YB, Roh KJ, Jung JA, Jung K, Yoo H, Cho YB, et al. Effect of SKI306X, a new herbal anti‐arthritic agent, in patients with osteoarthritis if the knee: A double‐blind placebo controlled study. American Journal of Chinese Medicine 2001;29(3‐4):485‐91. - PubMed
Jung 2004 {published data only}
-
- Jung YB, Seong SC, Lee MC, Shin YU, Kim DH, Kim JM, et al. A four‐week randomized, double‐blind trial of the efficacy and safety of SKI306X: A herbal anti‐arthritic agent versus diclofenac in osteoarthritis of the knee. American Journal of Chinese Medicine 2004;32(2):291‐301. - PubMed
Kimmatkar 2003 {published data only}
-
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee ‐ A randomized double blind placebo controlled trial. Phytomedicine 2003;10:3‐7. - PubMed
Kuptniratsaikul 2009 {published data only}
Kuptniratsaikul 2011 {published data only}
Leblan 2000 {published data only}
-
- Leblan D, Chantre P, Fournié B. Harpogophytum procumbens in the treatment of knee and hip osteoarthritis: Four‐month results of a prospective, multicenter, double‐blind trial versus diacerhein [L'harpagophyton dans le traitement de la gonarthrose et de la coxarthrose. Résultats à quatre mois d'une étude prospective multicentrique, contrôlée en double aveugle, versus diacerhéine]. Revue du Rhumatisme [Éd Fr Joint Bone Spine] 2000;67(5):462‐7. - PubMed
Lequesne 2002 {published data only}
-
- Lequesne M, Maheu E, Cadet C, Dreiser RL. Structural effect of avocado/soyabean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Care & Research 2002;47:50‐8. - PubMed
Maheu 1998 {published data only}
-
- Maheu E, Mazieres B, Valat J‐P, Loyau G, Loet X, Bourgeois P, et al. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip. Arthritis & Rheumatism 1998;41(1):81‐91. - PubMed
Maheu 2013 {published data only}
-
- Maheu E, Cadet C, Marty M, Moyse D, Kerloch I, Coste P, Dougados M, Mazières B, Spector TD, Halhol H, Grouin JM, Lequesne M. Randomised, controlled trial of avocado‐soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: the ERADIAS study. Annals of the Rheumatic Diseases 2013;Jan 23:[Epub ahead of print]. [DOI: 10.1136/annrheumdis-2012-202485] - DOI - PMC - PubMed
Majima 2012 {published data only}
Medhi 2009 {published data only}
Mehta 2007 {published data only}
-
- Mehta K, Gala J, Bhasale S, Naik S, Modak M, Thakur H, et al. Comparison of glucosamine sulfate and a polyherbal supplement for the relief of osteoarthritis of the knee: a randomized controlled trial [ISRCTN25438351]. BMC Complementary and Alternative Medicine 2007;7:34. [DOI: 10.1186/1472-6882-7-34] - DOI - PMC - PubMed
Mills 1996 {published data only}
-
- Mills SY, Jacoby RK, Chacksfield M, Willoughby M. Effect of a proprietary herbal medicine on the relief of chronic arthritic pain: A double‐blind study. British Journal of Rheumatology 1996;35:874‐8. - PubMed
Oben 2009 {published data only}
Pavelka 2010 {published data only}
Piscoya 2001 {published data only}
-
- Piscoya J, Rodriguez Z, Bustamante SA, Okuhama NN, Miller MJS, Sandoval M. Efficacy and safety of freeze‐dried cat's claw in osteoarthritis of the knee: Mechanism of action of the species Uncaria guianensis. Inflammation Research 2001;50:442‐8. - PubMed
Rein 2004a {published data only}
-
- Rein E, Kharazami A, Winther K. A herbal remedy, Hyben Vital (stand. powder of a subspecies of Rosa canina fruits), reduces pain and improves general wellbeing in patients with osteoarthritis ‐ a double‐blind, placebo‐controlled, randomised trial. Phytomedicine 2004;11:383‐91. - PubMed
Schadler 1988 {published data only (unpublished sought but not used)}
-
- Schadler W. Phytodolor for the treatment of activated arthrosis. Rheuma 1988;8:288‐90.
Schmelz 1997 {published data only}
-
- Schmelz H, Hammerle HD, Springorum HW. Analgesic effect of a Devil's claw extract in various chronic degenerative joint diseases [Analgetische Wirkung eines Teufelskrallenwurzel‐Extraktes bei verschiedenen chronisch‐degenerativen Gelenkerkrankungen]. In: Chrubasik S, Wink M editor(s). Rheumatherapie mit Phytopharmaka. Stuttgart: Hippokrates Verlag, 1997:86‐9.
Schmid 2000 {published data only}
-
- Schmid B, Ludtke R, Selbmann HK, Kotter I, Tschirdewahn B, Schaffner W, et al. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo‐controlled double blind clinical trial [Wirksamkeit und vertraglichkeit eines standardisierten weidenrindenextraktes bei arthose‐patienten: Randomisierte, placebo‐kontrollierte doppelblindstudie]. Zeitschrift für Rheumatologie 2000;59:1‐7. - PubMed
Sengupta 2008 {published data only}
Sengupta 2010 {published data only}
-
- Sengupta K, Krishnaraju AV, Vishal AA, Mishra A, Trimurtulu G, Sarma KVS, et al. Comparative efficacy and tolerability of 5‐Loxin® and Aflapin® against osteoarthritis of the knee: A double blind, randomized, placebo controlled clinical study. International Journal of Medical Sciences 2010;7(6):366‐77. - PMC - PubMed
Sontakke 2007 {published data only}
-
- Sontakke S, Thawani V, Pimpalkhute S, Kambra P, Babhulkar S, Hingorani L. Open, randomized, controlled trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian Journal of Pharmacology 2007;39:27‐9.
Teekachunhatean 2004 {published data only}
-
- Teekachunhatean S, Kunanusom P, Rojanasthien N, Sananpanich K, Pojchamarnwitputh S, Lheiochaiphunt S, Pruksakorn S. Chinese herbal recipe versus diclofenac in symptomatic treatment of osteoarthritis of the knee: A randomized controlled trial. BMC Comlementary and Alternative Medicine 2004;4:19. [DOI: 10.1186/1472‐6882‐4‐19] - PMC - PubMed
Vishal 2011 {published data only}
Warholm 2003 {published data only}
Wigler 2003 {published data only}
-
- Wigler I, Grotto I, Caspi D, Yaron M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthritis and Cartilage 2003;11:783‐9. - PubMed
Winther 2005 {published data only}
-
- Winther K, Apel K, Thamsborg G. A powder made from seeds and shells of a rose‐hip subspecies (Rosa canina) reduces symptoms of knee and hip osteoarthritis: A randomized, double‐blind, placebo‐controlled clinical trial. Scandinavian Journal of Rheumatology 2005;34:302‐8. - PubMed
References to studies excluded from this review
Anonymous 1993 {published data only}
-
- Anonymous. The garlic treatment: detoxifying with garlic. [Die knoblauchkur: entschlackung durch knoblauch]. Natur & Heilen 1993;70(10):520‐1.
Belcaro 2010 {published data only}
-
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, et al. Efficacy and safety of Meriva®, a curcumin‐phosphatidylcholine complex, during extended administration in osteoarthritis patients. Alternative Medicine Review 2010;15(4):337‐44. - PubMed
Biswas 1997 {published data only}
-
- Biswas NR, Biswas A, Pandey RM. EASE ‐ a herbal preparation for rheumatoid arthritis, non specific arthritis and osteoarthritis. FACT: Focus on Alternative and Complementary Therapies. Proceedings of 4th Annual Symposium on Complementary Health Care. Exeter (UK), 1997; Vol. 2, issue 4:186‐7.
Biswas 1998 {published data only}
-
- Biswas NR, Biswas K, Pandey M, Pandy RM. Treatment of osteoarthritis, rheumatoid arthritis and non‐specific arthritis with a herbal drug: a double‐blind, active drug controlled parallel study. JK Practitioner 1998;5(2):129‐32.
Brien 2006 {published data only}
-
- Brien S, Lewith GT, McGregor G. Devil's Claw (Harpagophytum procumbens) as a treatment for osteoarthritis: A review of safety and efficacy. The Journal of Alternative and Complementary Medicine 2006;12:981‐93. - PubMed
Chantre 2000 {published data only}
-
- Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000;7(3):177‐83. - PubMed
Chrubasik 1998 {published data only}
-
- Chrubasik S, Wink M. Traditional herbal therapy for the treatment of rheumatic pain: Preparations from Devil's claw and stinging nettle. Pain Digest 1998;8:94‐101.
Dharmananda 1985 {published data only}
-
- Dharmananda S. Chinese herbal therapies for chronic joint pain. The American Chiropractor 1985;January:38‐42.
Du 2006 {published data only}
-
- Du T‐X, Han X‐F, Gao S‐T. Effect of hanshibi granule on rheumatism due to blockage of cold and damp. Chinese Journal of Clinical Rehabilitation 2006;10:148‐50.
Falch 1997 {published data only}
-
- Falch VB. Ginger ‐ not only a spice. Review of its effect and efficacy profile [Ingwer ‐ nicht nur ein Gewurz. Untersuchungen zu Wirkungen und Wirksamkeit]. Deutsche Apotheker Zeitung 1997;137:47‐52, 55‐60.
Fang 2008 {published data only}
-
- Fang R, Meng Q‐C, Deng Y‐J, Song G, Bai Y‐P. Effects of Bu Shen Tong Luo decoction on matrix metalloproteinase‐1, matrix metalloproteinase‐3, tissue inhibitor of matrix metalloprotease‐1 and interleukin‐1beta content in the synovial fluid and serum of patients with knee osteoarthritis. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12:5581‐5.
Gendo 1997 {published data only}
-
- Gendo U. Chronische Polyarthritis in view of traditional Chinese medicine (TCM) [Die chronische Polyarthritis aus der Sicht der traditionellen Chinesischen Medizin (TCM)]. Naturamed 1997;12(10):37‐40.
Grahame 1981 {published data only}
Guyader 1984 {published data only}
-
- Guyader M. Herbal anti‐rheumatic therapies: A longitudinal, pharmacological and clinical study of the treatment of 50 osteoarthritic patients with Harpagophytum procumbens (Devil's Claw) following hospital intervention [Les plantes anti‐rhumatismales. Etude historique et pharmacologique, et etude clinique du nebulisat d'harpagophytum procumbens D.C. chez 50 patients arthrosiques suivis en service hospitalier]. Thesis for the award of the degree Doctor of Medicine. 1984.
Hamblin 2008 {published data only}
-
- Hamblin L, Laird A, Parkes E, Walker AF. Improved arthritic knee health in a pilot RCT of phytotherapy. The Journal of the Royal Society for the Promotion of Health 2008;128(5):255‐62. [CENTRAL: 00651201] - PubMed
Jacquet 2009 {published data only}
-
- Jacquet A, Girodet P, Pariente A, Forest K, Mallet, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double‐blind placebo‐controlled clinical trial. Arthritis Research & Therapy 2009;11:R192. [DOI: 10.1186/ar2891] - DOI - PMC - PubMed
Kagore 2011 {published data only}
-
- Kogure T, Tatsumi T, Shigeta T, Fujinaga H, Sato T, Niizawa A. Effect of Kampo medicine on pain and range of motion of osteoarthritis of the hip accompanied by acetabular dysplasia: Case report and literature review. Integrative Medicine Highlights 2011;6:13‐7. [DOI: 10.4137/IMI.S7884] - DOI - PMC - PubMed
Kielczynski 1997 {published data only}
-
- Kielczynski W. Osteoarthritis ‐ clinical outcomes after uniform, long‐term herbal treatment. The European Journal of Herbal Medicine 1997;3(2):29‐35.
Kulkarni 1991 {published data only}
-
- Kulkarni RR, Patki PS, Jog VP, Gandage SG, Patwardhan B. Treatment of osteoarthritis with a herbomineral formulation: A double‐blind, placebo‐controlled, cross‐over study. Journal of Ethnopharmacology 1991;33:91‐5. - PubMed
Lechner 2011 {published data only}
-
- Lechner M, Steirer I, Brinkhaus B, Chen Y, Krist‐Dungl C, Koschier A, et al. Efficacy of individualized Chinese herbal medication in osteoarthrosis of hip and knee: a double‐blind, randomized‐controlled clinical study. Journal of Alternative and Complementary Medicine 2011;17(6):539‐47. - PubMed
Levy 2009 {published data only}
-
- Levy RM, Saikovsky R, Shmidt E, Khokhlov A, Burnett BP. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short‐term randomized, double‐blind pilot study. Nutrition Research 2009;29:298‐304. - PubMed
Linsheng 1997 {published data only}
-
- Linsheng W. Treatment of bony arthritis with herbal medicine and by massotherapy ‐ analysis of 121 cases. Journal of Traditional Chinese Medicine 1997;17(1):32‐6. - PubMed
Loew 1996 {published data only}
-
- Loew D, Schuster O, Mollerfeld J. Stability and biopharmaceutical quality. A postulate for bioavailability and effectiveness of Harpagophytum procumbens [Stabilitat und biopharmazeutische Qualitat. Voraussetzung fur Bioverfugbarkeit und Wirksamkeit von Harpagophytum procumbens]. In: Loew D, Rietcrock N editor(s). Phytopharmaka II. Forschung und klinische Anwendung. Darmstadt: Steinkopf‐Verlag, 1996:83‐93.
Long 2001 {published data only}
-
- Long L, Soeken K, Ernst E. Herbal medicines for the treatment of osteoarthritis: A systematic review. Rheumatology 2001;40:779‐93. - PubMed
Lung 2004 {published data only}
-
- Lung YB, Seong SC, Lee MC, Shin YU, Kim DH, Kim JM, et al. A four‐week, randomized, double‐blind trial of the efficacy and safety of SKI306X: a herbal anti‐arthritic agent versus diclofenac in osteoarthritis of the knee. The American Journal of Chinese Medicine 2004;32:291‐301. - PubMed
Mishra and Singh 2003 {published data only}
-
- Mishra LC, Singh BB, Vinjamury SP. The effectiveness of Commiphora mukul for osteoarthritis of the knee: an outcomes study. Althernative Therapies in Health and Medicine 2003;9:74‐9. - PubMed
Myers 2010 {published data only}
Park 2009 {published data only}
Rein 2004b {published data only}
-
- Rein E, Kharazmi A, Thamsborg G, Winther K. A herbal remedy, made from a subspecies of rose‐hip Rosa canina, reduces symptoms of knee and hip osteoarthritis. Osteoarthritis and Cartilage 2004;12 Suppl 2:S80.
Reuss 1981 {published data only}
-
- Ruess D. Echinacin in the treatment of primary chronic polyarthritis [Echinacin in der Therapie der primar‐chronischen Polyarthritis]. ZFA ‐ Stuttgart 1981;57(11):865. - PubMed
Rosen 2013 {published data only}
-
- Rosen PD, Liakeas GP, Sadigim E, Bied AM. A pilot study assessing the short term use of Tanacetum parthenium for treatment of osteoarthritis. Integrative Medicine 2013;12(3):31‐6.
Sagar 1988 {published data only}
-
- Sagar VMV. A clinical study of Amavata with special reference to some indigenous drugs. Rheumatism 1988‐89;24(3):3‐7.
Saley 1987 {published data only}
-
- Saley SR, Tilak MN, Deshmukh SS. Amavata ‐ a clinical study of 41 cases. Rheumatism 1987;22(2):46‐50.
Schaffner 1997 {published data only}
-
- Schaffner W. Willow bark ‐ an antirheumatic in modern phytotherapy? [Weidenrinde ‐ Ein Antirheumatikum der modernen Phytotherapie?]. In: Chrubasik S, Wink M editor(s). Rheumatherapie mit Phytopharmaka. Stuttgart: Hippokrates Verglag, 1997:125‐7.
Schmid 1998a {published data only}
-
- Schmid B, Tschirdewahn B, Kotter I, Gunaydin I, Ludtke R, Selbmann HK, et al. Analgesic effects of willow bark extract in osteoarthritis: results of a clinical double‐blind trial. FACT:Focus on Alternative and Complementary Therapies 1998;3(4):186.
Schmid 2001 {published data only}
-
- Schmid B, Lüdtke R, Selbmann H‐K, Kötter I, Tschirdewahn B, Schaffner W, Heide L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo‐controlled double blind clinical trial. Phytotherapy Research 2001;15:344‐50. [DOI: 10.1002/ptr.981] - DOI - PubMed
Srivastava 1989 {published data only}
-
- Srivastava KC, Mustafa T. Ginger (Zingiber officinale) and rheumatic disorders. Medical Hypotheses 1989;29:25‐8. - PubMed
Wang 1985 {published data only}
-
- Wang ZM, Chang JY, et al. A report on 310 cases of articular rheumatism treated with Feng Shi Han Tong tablet. Chung Hsi I Chieh Ho Tsa Chih 1985;5(5):284‐5, 260. - PubMed
Wegener 2003 {published data only}
-
- Wegener T, Lupke NP. Treatment of patients with arthrosis of hip or knee with an aqueous extract of Devil's claw (Harpagophytum procumbens DC). Phytotherapy Research 2003;17(10):1165‐72. - PubMed
Winther 2004 {published data only}
-
- Winther K, Kharazmi A, Rein E. A powder made from a subspecies of rosehip (Rosa canina) reduces WOMAC symptoms scores as well as cholesterol levels in patients suffering from osteoarthritis. Osteoarthritis and Cartilage 2004;13 Suppl A:S93.
Xu 2005 {published data only}
-
- Xu J‐W, Ding J‐X. An analysis on the clinical therapeutic effect of the manipulation matched with Chinese medical herb washing for treatment of osteoarthritis of the knee. Journal of Beijing Teachers College of Physical Education 2005;1:9.
Yuelong 2011 {published data only}
Zell 1993 {published data only}
-
- Zell J, Ecker R, Batz H, Vestweber AM. Mistletoe for hip and knee arthrosis: Segment ‐ or immunotherapy? [Misteltherapie bei Gon‐ und Coxarthrose: Segment ‐ oder Immuntherapie? Eine Pilotstudie]. Heilkunst 1993;106(9):23‐6.
Zeng 2008 {published data only}
-
- Zeng Y‐R, Fan Y‐G, Wu F, Li X‐P, Fan H‐J. Effects of compound herb of invigorating kidney and promoting blood flow on serum matrix metalloproteinases‐3, tumor necrosis factor alpha, interleukin‐1, hyaluronic acid, lipid peroxidation levels and superoxide dismutase activity in patients with knee osteoarthritis. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12:5436‐9.
References to studies awaiting assessment
Gao 2012 {published data only}
-
- Gao G, Wu H, Tian J, Du J, Xie X, Gao J. Clinical efficacy of bushen huoxue qubi decoction on treatment of knee‐osteoarthritis and its effect on hemarheology, anti‐inflammation and antioxidation. Zhongguo Zhong Yao Za Zhi 2012;37(3):390‐6. - PubMed
Hochberg 2012 {published data only}
-
- Hochberg MC, Lao LX, Langenberg P, Fong HHS, Lee DYW, Berman B. Efficacy and Safety of the Chinese Herbal Compound Hou‐Lou‐Xiao‐Ling Dan in Patients with Osteoarthritis of the Knee: Results of a Phase II International Study [Abstract]. Arthritis and Rheumatism 2012;64(10):S112. [DOI: 10.1002/art.37993] - DOI
Kang 2011 {published data only}
-
- Kang XZ, Wu QF, Jie HY. Clinical study on the treatment of knee osteoarthritis by wangbi tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chinese Journal of integrated Traditional and Western Medicine] 2011;31(9):1205‐8. - PubMed
Liu 2006 {published data only}
-
- Liu H‐B, Zhang J‐F, Zhong L‐W, Yang H. Manipulation plus pyrola compound traditional Chinese medicine in treatment of senile osteoarthritis of knee. Chinese Journal of Clinical Rehabilitation 2006;10(23):30‐2. [CENTRAL: 00613036]
Pinsornsak 2012 {published data only}
-
- Pinsornsak P, Niempoog S. The efficacy of Curcuma longa L. extract as an adjuvant therapy in primary knee osteoarthritis: A randomized controlled trial. Journal of the Medical Association of Thailand 2012;95 Suppl 1:S51‐8. - PubMed
Tao 2009 {published data only}
-
- Tao QW, Xu Y, Jin DE, Yan XP. Clinical efficacy and safety of Gubitong Recipe () in treating osteoarthritis of knee joint. Chinese Journal of Integrative Medicine 2009;15(6):458‐61. - PubMed
Zhong 2006 {published data only}
-
- Zhong Q‐S, Ye G‐H, Wang H‐Z, Lin S‐H. Treatment of knee osteoarthritis by invigorating the kidney, dispelling the cold and activating the collaterals: A randomized controlled study. Chinese Journal of Clinical Rehabilitation 2006;10:177‐9.
Additional references
Altman 1986
-
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. The American College of Rheumatology crtieria for the classification and reporting of osteoarthritis of the knee. Arthritis & Rheumatism 1986;29:1039‐49. - PubMed
Altman 1990
-
- Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis & Rheumatism 1990;33:1601‐10. - PubMed
Altman 1991
-
- Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis & Rheumatism 1991;34:505‐14. - PubMed
Basch 2004
-
- Basch E, Boon H, Davies‐Heerema T, Foppo I, Hashmi S, Hasskarl J, et al. Boswellia: an evidence‐based systematic review by the Natural Standard Research Collaboration. Journal of Herbal Pharmacotherapy 2004;4(3):63‐83. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horeton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT Statement. JAMA 1996;276(8):637‐9. - PubMed
Blumenthal 1998
-
- Blumenthal M. The complete German Commission E Monographs. Austin, TX: American Botanical Council, 1998.
Boullata 2003
-
- Boullata JI, McDonnell PJ, Oliva CD. Anaphylactic reaction to a dietary supplement containing willow bark. Annals of Pharmacotherapy 2003;37:832‐5. - PubMed
Cameron 2011
Cameron 2013
Cates 2008
-
- Cates CD. Visual Rx NNT Calculator. Dr Chris Cates EBM website. Available from http://www.nntonline.net/, 2008.
Choi 2002
-
- Choi JH, Choi JH, Kim DY, Yoon JH, Youn HY, Yi JB, et al. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase‐induced rabbit osteoarthritis model. Osteoarthritis and Cartilage 2002;10:471‐8. - PubMed
Christensen 2008a
-
- Christensen R, Bartels EM, Astrup A, Bliddal H. Symptomatic efficacy of avocado‐soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: a meta‐analysis of randomized controlled trials.. Osteoarthritis and Cartilage 2008;16:399‐408. - PubMed
Christensen 2008b
-
- Christensen R, Bartels EM, Altman RD, Astrup A, Bliddal H. Does the hip powder of Rosa canina (rosehip) reduce pain in osteoarthritis patients? A meta‐analysis of randomized controlled trials. Osteoarthritis and Cartilage 2008;16:965‐72. - PubMed
Chrubasik 1996
-
- Chrubasik S, Sporer F, Wink M. Active ingredients in medicines from Harpagophytum procumbens [Zum Wirkstoffgehalt in Arzneimitteln aus Harpagophytum procumbens]. Forschende Komplementarmedizin 1996;3:57‐63.
Chrubasik 1999
-
- Chrubasik S, Junck H, Breitschwerdt H, Conradt C, Zappe H. Effectiveness of Harpagophytum extract WS 1531 in the treatment of exacerbation of low back pain: A randomized, placebo‐controlled, double‐blind study. European Journal of Anaesthesiology 1999;16:118‐29. - PubMed
Chrubasik 2000
-
- Chrubasik S, Eisenberg E, Balan E, Weinberger T, Luzzati R, Conradt C. Treatment of low back pain exacerbations with willow bark extract: A randomized double‐blind study. American Journal of Medicine 2000;109:9‐14. - PubMed
Chrubasik 2001
-
- Chrubasik S, Künzel O, Model A, Conradt C, Black A. Treatment of low back pain with a herbal or syntheticantirheumatic: a randomized controlled study. Rheumatology 2001;40:1388‐93. - PubMed
Chrubasik 2002
-
- Chrubasik S, Fiebich B, Black A, Pollak S. Treating low back pain with an extract of Harpagophytum that inhibits cytokine release. European Journal of Anaesthesiology 2002;19:209.
Chrubasik 2003
-
- Chrubasik S, Roufogalis B. Bioequivalence of herbal medicines. New Zealand Journal of Pharmacy 2003;53:39‐44.
Chrubasik 2005
-
- Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005;12(9):684‐701. - PubMed
Chrubasik 2006
-
- Chrubasik JE, Lindhorst E, Neumann E, Gerlach U, Faller‐Marquardt M, Torda T, Muller‐Ladner U, Chrubasik S. Potential molecular basis of the chondroprotective effect of Harpagophytum procumbens. Phytomedicine 2006;13:598‐600. - PubMed
Chrubasik 2007
-
- Chrubasik JE, Roufogalis BD, Chrubasik S. Evidence of effectiveness of herbal anti‐inflammatory drugs in the treatment of painful osteoarthritis including chronic low back pain. Phytotherapy Research 2007;21:675‐83. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
EMA 2009
-
- European Medicines Agency (EMA). Assessment report on salicis cortex (willow bark) and herbal preparation(s) thereof with well‐established use and traditional use. EMA, London, 2009 2009.
ESCOP 2003
-
- European Scientific Cooperative on Phytotherapy (Eds). ESCOP Monographs. New York: Thieme‐Verlag Stuttgart, 2003.
ESCOP 2009
-
- European Scientific Cooperative on Phytotherapy (Eds). ESCOP Monographs. Vol. Supplement, Stuttgart, New York: Thieme Press, 2009.
Gabriel 1991
-
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti‐inflammatory drugs. A meta‐analysis. Annals of Internal Medicine 1991;115:787‐96. - PubMed
Glinko 1998
-
- Glinko A. Pharmacological properties of a standardized extract from Willow Bark (Cortex salicis) [treatise]. Chair of Pharmacology and Toxicology. Pomeranian Academy of Medicine. Szczecin, 1998.
Gundermann 2001
-
- Gundermann KJ. Phytodolor solution. Clinical expert report. Darmstadt, Germany: Steigerwald, 2001.
Hadhyiski 2006
-
- Hadzhiyski H, Lindhorst E, Raif W, Torda T, Chrubasik S. Impact of Harpagophytum procumbens on the urinary pyridinoline deoxypyridinoline ratio in experimental osteoarthritis [Abstract]. FACT: Focus on Alternative and Complementary Therapies 2006;11:13.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies.. Higgins JPT, Green S (editors). The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011.
ICH 2004
-
- ICH. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Synopsis of ICH guidelines and topics. Geneva, Switzerland: Author, 2004.
Jäger 2007
-
- Jäger AK, Eldeen IM, Staden J. COX‐1 and ‐2 activity of rose hip. Phytotherapy Research 2007;21(12):1251‐2. - PubMed
Jäger 2008
-
- Jäger AK, Petersen KN, Thomasen G, Christensen SB. Isolation of linoleic and alpha‐linolenic acids as COX‐1 and ‐2 inhibitors in rose hip. Phytotherapy Research 2008;22(7):982‐4. - PubMed
Krishnaraju 2010
-
- Krishnaraju AV, Sundararaju D, Vamsikrishna U, Suryachandra R, Machiraju G, Sengupta K, Trimurtulu G. Safety and toxicological evaluation of Aflapin: a novel Boswellia‐derived anti‐inflammatory product. Toxicology Mechanisms and Methods 2010;20(9):556‐63. - PubMed
Krivoy 2001
-
- Krivoy N, Pavlotzky E, Chrubasik S, Eisenberg E, Brooks G. Effect of Salicis cortex extract on human platelet aggregation. Planta Medica 2001;67:209‐13. - PubMed
Lawrence 2008
Lee 2010
-
- Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta‐analysis. Rheumatology International 2010;30(3):357‐63. - PubMed
Malemud 2010
Mazieres 1993
-
- Mazieres B, Tempesta C, Tiechard M, Vaguier G. Pathologic and biochemical effects of a lipidic avocado and soya extract on an experimental post‐contusive model of OA [Abstract]. Osteoarthritis and Cartilage 1993;1:46.
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134:657‐62. - PubMed
Ofman 2002
-
- Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, Shekelle P. A metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. Journal of Rheumatology 2002;29:804‐12. - PubMed
Ofman 2003
-
- Ofman JJ, Maclean CH, Straus WL, Morton SC, Berger ML, Roth EA, Shekelle PG. Meta‐analysis of dyspepsia and nonsteroidal antiinflammatory drugs. Arthritis & Rheumatism 2003;49:508‐18. - PubMed
Olivier 2010
-
- Olivier P, Montastruc JL. Réseau français des centres régionaux de pharmacovigilance [Post‐marketing safety profile of avocado‐soybean unsaponifiables]. Presse Medicale 2010;39(10):e211‐6. - PubMed
Pham 2003
-
- Pham T, Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: The OMERACT‐OARSI set of responder criteria. Journal of Rheumatology 2003;30:1645‐54. - PubMed
Pham 2004
-
- Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and Cartilage 2004;12:389‐99. - PubMed
Reginister 2007
Reichenbach 2007
-
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Bürgi E, Bürgi U, et al. Meta‐analysis: chondroitin for osteoarthritis of the knee or hip. Annals of Internal Medicine 2007;146(8):580‐90. - PubMed
Sangha 2000
-
- Sangha O. Epidemiology of rheumatic diseases. Rheumatology 2000;39 Suppl 2:3‐12. - PubMed
Schmid 1998b
-
- Schmid B. Treatment of hip and knee osteoarthritis with willow bark extract [Behandlung von Cox‐ und Gonarthrosen mit einem Weidenrindenextrakt]. Dissertation Universität Tübingen 1998.
Schmitz 2010
-
- Schmitz N, Kraus VB, Aigner T. Targets to tackle‐‐the pathophysiology of the disease. Current Drug Targets 2010;11:521‐7. - PubMed
Schoonees 2012
Schunemann 2011a
-
- Schunemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, updated March 2011.
Schunemann 2011b
-
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, updated March 2011.
Smalley 1996
-
- Smalley WE, Griffin MR, Fought RL, Ray WA. Excess costs from gastrointestinal disease associated with nonsteroidal anti‐inflammatory drugs. Journal of General Internal Medicine 1996;11:461‐9. - PubMed
Sporer 1999
-
- Sporer F, Chrubasik S. Preparations from Devil's claw (Harpagophytum procumbens) [Präparate aus der Teufelskralle (Harpagophytum procumbens)]. Zeitschrift für Phytotherapie 1999;20:235‐6.
Stohs 2011
Stone 1763
-
- Stone E. An account of the success of the bark of the willow in the cure of agues. Philosophy. Trans: Royal Society London 1763;53:195‐200.
Valerio 2005
-
- Valerio LG Jr, Gonzales GF. Toxicological aspects of the South American herbs cat's claw (Uncaria tomentosa) and Maca (Lepidium meyenii) : a critical synopsis. Toxicology Review 2005;24(1):11‐35. - PubMed
van den Berg 2011
-
- Berg WB. Osteoarthritis year 2010 in review: Pathomechanisms. Osteoarthritis and Cartilage 2011;19:338‐41. - PubMed
Vlachojannis 2008
Vlachojannis 2009
-
- Vlachojannis JE, Duke RK, Tran VH, Duke CC, Chrubasik S. Anti‐inflammatory herbal medicines for the control of pain. In: Carpinella M C, Rai M editor(s). Novel Therapeutic Agents from Plants. Enfield, NH, USA: Science Publisher, 2009:339‐67.
Vlachojannis 2013
-
- Vlachojannis C, Magora F, Chrubasik S. Pro and contra duration restriction of willow bark treatment. Phytotherapy Research 2014;28(1):148‐9. - PubMed
Wandel 2010
Wenzig 2008
-
- Wenzig EM, Widowitz U, Kunert O, Chrubasik S, Bucar F, Knauder E, Bauer R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008;15(10):826‐35. - PubMed
Zhang 2009
-
- Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence based recommendations for the diagnosis of hand osteoarthritis ‐ report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of Rheumatic Diseases 2009;68:8‐17. - PubMed
Zhang 2010a
Zhang 2010b
-
- Zhang W, Doherty M, Peat G, Bierma‐Zeinstra MA, Arden NK, Bresnihan B, et al. EULAR evidence‐based recommendations for the diagnosis of knee osteoarthritis. Annals of Rheumatic Diseases 2010;69(3):483‐9. - PubMed
References to other published versions of this review
Cameron 2007
-
- Cameron M, Chrubasik S, Parsons T, Gagnier J, Blümle A, Little C. Herbal therapy for treating osteoarthritis: Update of a Cochrane review. Annals of the Rheumatic Diseases 2007;66 Supp II:494.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

