Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach
- PMID: 24848762
- PMCID: PMC4179650
- DOI: 10.1208/s12249-014-0137-4
Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach
Abstract
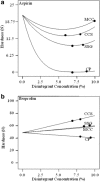
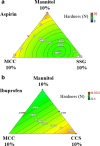
Investigation of the effect of disintegrants on the disintegration time and hardness of rapidly disintegrating tablets (RDTs) was carried out using a quality by design (QbD) paradigm. Ascorbic acid, aspirin, and ibuprofen, which have different water solubilities, were chosen as the drug models. Disintegration time and hardness of RDTs were determined and modeled by executing combined optimal design. The generated models were validated and used for further analysis. Sodium starch glycolate, croscarmellose sodium, and crospovidone were found to lengthen disintegration time when utilized at high concentrations. Sodium starch glycolate and crospovidone worked synergistically in aspirin RDTs to decrease disintegration time. Sodium starch glycolate-crospovidone mixtures, as well as croscarmellose sodium-crospovidone mixtures, also decreased disintegration time in ibuprofen RDTs at high compression pressures as compared to the disintegrants used alone. The use of sodium starch glycolate in RDTs with highly water soluble active ingredients like ascorbic acid slowed disintegration, while microcrystalline cellulose and crospovidone drew water into the tablet rapidly and quickened disintegration. Graphical optimization analysis demonstrated that the RDTs with desired disintegration times and hardness can be formulated with a larger area of design space by combining disintegrants at difference compression pressures. QbD was an efficient and effective paradigm in understanding formulation and process parameters and building quality in to RDT formulated systems.
Figures






References
-
- Food and Drug Administration. Guidance for industry. Orally disintegrating tablets. Silver Spring: US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

