Mu-opioid receptor splice variants: sex-dependent regulation by chronic morphine
- PMID: 24848866
- PMCID: PMC4156545
- DOI: 10.1111/jnc.12768
Mu-opioid receptor splice variants: sex-dependent regulation by chronic morphine
Abstract
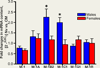
The gene encoding the mu-opioid receptor (MOR) generates a remarkable diversity of subtypes, the functional significance of which remains largely unknown. The structure of MOR could be a critical determinant of MOR functionality and its adaptations to chronic morphine exposure. As MOR antinociception has sexually dimorphic dimensions, we determined the influence of sex, stage of estrus cycle, and chronic systemic morphine on levels of MOR splice variant mRNA in rat spinal cord. Chronic systemic morphine influenced the spinal expression of mRNA encoding rMOR-1B2 and rMOR-1C1 in a profoundly sex-dependent fashion. In males, chronic morphine resulted in a twofold increase in expression levels of rMOR-1B2 and rMOR-1C1 mRNA. This effect of chronic morphine was completely absent in females. Increased density of MOR protein in spinal cord of males accompanied the chronic morphine-induced increase in MOR variant mRNA, suggesting that it reflected an increase in corresponding receptor protein. These results suggest that tolerance/dependence results, at least in part, from different adaptational strategies in males and females. The signaling consequences of the unique composition of the C-terminus tip of rMOR-1C1 and rMOR-1B2 could point the way to defining the molecular components of sex-dependent tolerance and withdrawal mechanisms. Chronic systemic morphine increases levels of mRNA encoding two splice variants of mu-opioid receptor (MOR), MOR-1B2 and MOR-1C1, variants differing from rMOR-1 in their C-terminal (and phosphorylation sites therein) and thus possibly signaling sequelae. This adaptation is sex-specific. It occurs in the spinal cord of males, but not females, indicating the importance of sex-specific mechanisms for and treatments of tolerance and addiction.
Keywords: mu-opioid receptor; opioid tolerance; sexual dimorphism; splice variants.
© 2014 International Society for Neurochemistry.
Figures

References
-
- Abbadie C, Gultekin SH, Pasternak GW. Immunohistochemical localization of the carboxy terminus of the novel mu opioid receptor splice variant MOR-1C within the human spinal cord. Neuroreport. 2000a;11:1953–1957. - PubMed
-
- Abbadie C, Pan Y, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience. 2000b;100:141–153. - PubMed
-
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000c;419:244–256. - PubMed
-
- Abbadie C, Pasternak GW. Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport. 2001;12:3069–3072. - PubMed
-
- Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol. 2002;452:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

