CT angiography after 20 years: a transformation in cardiovascular disease characterization continues to advance
- PMID: 24848958
- PMCID: PMC4669887
- DOI: 10.1148/radiol.14132232
CT angiography after 20 years: a transformation in cardiovascular disease characterization continues to advance
Abstract
Through a marriage of spiral computed tomography (CT) and graphical volumetric image processing, CT angiography was born 20 years ago. Fueled by a series of technical innovations in CT and image processing, over the next 5-15 years, CT angiography toppled conventional angiography, the undisputed diagnostic reference standard for vascular disease for the prior 70 years, as the preferred modality for the diagnosis and characterization of most cardiovascular abnormalities. This review recounts the evolution of CT angiography from its development and early challenges to a maturing modality that has provided unique insights into cardiovascular disease characterization and management. Selected clinical challenges, which include acute aortic syndromes, peripheral vascular disease, aortic stent-graft and transcatheter aortic valve assessment, and coronary artery disease, are presented as contrasting examples of how CT angiography is changing our approach to cardiovascular disease diagnosis and management. Finally, the recently introduced capabilities for multispectral imaging, tissue perfusion imaging, and radiation dose reduction through iterative reconstruction are explored with consideration toward the continued refinement and advancement of CT angiography.
Conflict of interest statement
Figures

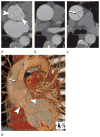

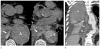

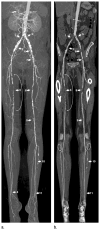
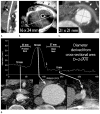
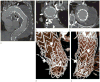


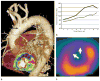
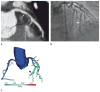
References
-
- Brooks B. Intra-arterial injection of sodium iodid: preliminary report. JAMA. 1924;82(13):1016–1019.
-
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39(5 Suppl):368–376. - PubMed
-
- Dumoulin CL, Hart HR., Jr Magnetic resonance angiography in the head and neck. Acta Radiol Suppl. 1986;369:17–20. - PubMed
-
- Nishimura DG, Macovski A, Pauly JM. Magnetic resonance angiography. IEEE Trans Med Imaging. 1986;5(3):140–151. - PubMed
-
- Wing VW, Federle MP, Morris JA, Jr, Jeffrey RB, Bluth R. The clinical impact of CT for blunt abdominal trauma. AJR Am J Roentgenol. 1985;145(6):1191–1194. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

