Pharmacokinetics of a prototype formulation of sublingual testosterone and a buspirone tablet, versus an advanced combination tablet of testosterone and buspirone in healthy premenopausal women
- PMID: 24849043
- PMCID: PMC4070462
- DOI: 10.1007/s40268-014-0047-7
Pharmacokinetics of a prototype formulation of sublingual testosterone and a buspirone tablet, versus an advanced combination tablet of testosterone and buspirone in healthy premenopausal women
Abstract
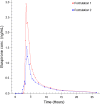
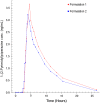
The study aimed to compare the kinetics of two novel combination drug products for Female Sexual Interest/Arousal Disorder (FSIAD). Thirteen women received testosterone via the sublingual route followed 2.5 hours later by a buspirone tablet, versus a single combination tablet swallowed at once. The first clinical prototype consisted of a sublingual solution containing testosterone (0.5 mg) complexed with cyclodextrin and a tablet containing 10 mg buspirone, in a gelatin capsule to ensure blinding during the clinical studies. The innovative fixed-combination tablet consists of an inner-core component of 10 mg buspirone coated with a polymeric time-delay coating and an outer polymeric coating containing testosterone with hydroxypropyl-beta cyclodextrin. We observed an immediate testosterone pulse absorption from both formulations. We also demonstrated that there was adequate absorption of buspirone (>80 % relative to the conventional tablet) and a time delay in release of buspirone of 3.3 hours, close to the 3.0 hours of the reference formulation that showed clinical efficacy in early proof-of-principle studies. The newly developed combination tablet fulfils its design criteria and is a convenient tablet for further clinical studies in FSIAD.
Figures



Similar articles
-
Single dose sublingual testosterone and oral sildenafil vs. a dual route/dual release fixed dose combination tablet: a pharmacokinetic comparison.Br J Clin Pharmacol. 2016 Jun;81(6):1091-102. doi: 10.1111/bcp.12887. Epub 2016 Mar 7. Br J Clin Pharmacol. 2016. PMID: 26804967 Free PMC article. Clinical Trial.
-
The Effect of Food on the Pharmacokinetics of Buspirone After Single Administration of a Sublingual Testosterone and Oral Buspirone Combination Tablet in Healthy Female Subjects.Sex Med. 2020 Jun;8(2):186-194. doi: 10.1016/j.esxm.2020.01.005. Epub 2020 Feb 20. Sex Med. 2020. PMID: 32088143 Free PMC article.
-
The Effect of Food on the Pharmacokinetics of Sildenafil after Single Administration of a Sublingual Testosterone and Oral Sildenafil Combination Tablet in Healthy Female Subjects.J Sex Med. 2019 Sep;16(9):1433-1443. doi: 10.1016/j.jsxm.2019.06.015. J Sex Med. 2019. PMID: 31488289 Clinical Trial.
-
Efficacy and Safety of On-Demand Use of 2 Treatments Designed for Different Etiologies of Female Sexual Interest/Arousal Disorder: 3 Randomized Clinical Trials.J Sex Med. 2018 Feb;15(2):201-216. doi: 10.1016/j.jsxm.2017.11.226. Epub 2017 Dec 27. J Sex Med. 2018. PMID: 29289554 Clinical Trial.
-
Pharmacokinetics of buspirone extended-release tablets: a single-dose study.J Clin Pharmacol. 2001 Jul;41(7):783-9. doi: 10.1177/00912700122010582. J Clin Pharmacol. 2001. PMID: 11452712 Clinical Trial.
Cited by
-
Single dose sublingual testosterone and oral sildenafil vs. a dual route/dual release fixed dose combination tablet: a pharmacokinetic comparison.Br J Clin Pharmacol. 2016 Jun;81(6):1091-102. doi: 10.1111/bcp.12887. Epub 2016 Mar 7. Br J Clin Pharmacol. 2016. PMID: 26804967 Free PMC article. Clinical Trial.
-
Genotype scores predict drug efficacy in subtypes of female sexual interest/arousal disorder: A double-blind, randomized, placebo-controlled cross-over trial.Womens Health (Lond). 2018 Jan-Dec;14:1745506518788970. doi: 10.1177/1745506518788970. Womens Health (Lond). 2018. PMID: 30016917 Free PMC article. Clinical Trial.
-
A survival of the fittest strategy for the selection of genotypes by which drug responders and non-responders can be predicted in small groups.PLoS One. 2021 Mar 5;16(3):e0246828. doi: 10.1371/journal.pone.0246828. eCollection 2021. PLoS One. 2021. PMID: 33667227 Free PMC article. Clinical Trial.
-
Current and future pharmacotherapy for female sexual dysfunction.Nat Rev Urol. 2025 Aug 20. doi: 10.1038/s41585-025-01076-w. Online ahead of print. Nat Rev Urol. 2025. PMID: 40836011 Review.
-
The Effect of Food on the Pharmacokinetics of Buspirone After Single Administration of a Sublingual Testosterone and Oral Buspirone Combination Tablet in Healthy Female Subjects.Sex Med. 2020 Jun;8(2):186-194. doi: 10.1016/j.esxm.2020.01.005. Epub 2020 Feb 20. Sex Med. 2020. PMID: 32088143 Free PMC article.
References
-
- Fugl-Meyer KS. Sexual disabilities and sexual problems. In: Sex in Sweden. Stockholm: National Institute of Public Health; 2000. pp. 199–216.
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

