The role of intracellular linkers in gating and desensitization of human pentameric ligand-gated ion channels
- PMID: 24849357
- PMCID: PMC4028499
- DOI: 10.1523/JNEUROSCI.5105-13.2014
The role of intracellular linkers in gating and desensitization of human pentameric ligand-gated ion channels
Abstract
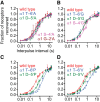
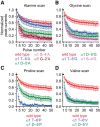
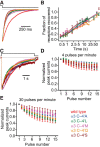
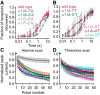
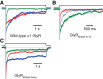
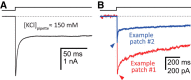
It has recently been proposed that post-translational modification of not only the M3-M4 linker but also the M1-M2 linker of pentameric ligand-gated ion channels modulates function in vivo. To estimate the involvement of the M1-M2 linker in gating and desensitization, we engineered a series of mutations to this linker of the human adult-muscle acetylcholine receptor (AChR), the α3β4 AChR and the homomeric α1 glycine receptor (GlyR). All tested M1-M2 linker mutations had little effect on the kinetics of deactivation or desensitization compared with the effects of mutations to the M2 α-helix or the extracellular M2-M3 linker. However, when the effects of mutations were assessed with 50 Hz trains of ∼1 ms pulses of saturating neurotransmitter, some mutations led to much more, and others to much less, peak-current depression than observed for the wild-type channels, suggesting that these mutations could affect the fidelity of fast synaptic transmission. Nevertheless, no mutation to this linker could mimic the irreversible loss of responsiveness reported to result from the oxidation of the M1-M2 linker cysteines of the α3 AChR subunit. We also replaced the M3-M4 linker of the α1 GlyR with much shorter peptides and found that none of these extensive changes affects channel deactivation strongly or reduces the marked variability in desensitization kinetics that characterizes the wild-type channel. However, we found that these large mutations to the M3-M4 linker can have pronounced effects on desensitization kinetics, supporting the notion that its post-translational modification could indeed modulate α1 GlyR behavior.
Keywords: fast perfusion; glycine receptors; ion-channel kinetics; nicotinic receptors; outside-out patches; patch-clamp.
Copyright © 2014 the authors 0270-6474/14/347238-15$15.00/0.
Figures





References
-
- Amici SA, McKay SB, Wells GB, Robson JI, Nasir M, Ponath G, Anand R. A highly conserved cytoplasmic cysteine residue in the alpha4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression. J Biol Chem. 2012;287:23119–23127. doi: 10.1074/jbc.M111.328294. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
