Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses
- PMID: 24849359
- PMCID: PMC6608192
- DOI: 10.1523/JNEUROSCI.3973-13.2014
Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses
Abstract
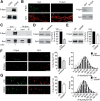
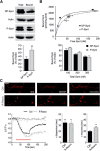
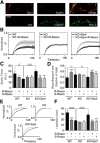
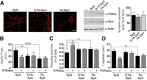
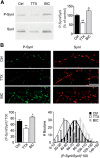
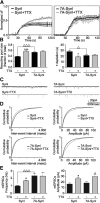
Cyclin-dependent kinase-5 (Cdk5) was reported to downscale neurotransmission by sequestering synaptic vesicles (SVs) in the release-reluctant resting pool, but the molecular targets mediating this activity remain unknown. Synapsin I (SynI), a major SV phosphoprotein involved in the regulation of SV trafficking and neurotransmitter release, is one of the presynaptic substrates of Cdk5, which phosphorylates it in its C-terminal region at Ser(549) (site 6) and Ser(551) (site 7). Here we demonstrate that Cdk5 phosphorylation of SynI fine tunes the recruitment of SVs to the active recycling pool and contributes to the Cdk5-mediated homeostatic responses. Phosphorylation of SynI by Cdk5 is physiologically regulated and enhances its binding to F-actin. The effects of Cdk5 inhibition on the size and depletion kinetics of the recycling pool, as well as on SV distribution within the nerve terminal, are virtually abolished in mouse SynI knock-out (KO) neurons or in KO neurons expressing the dephosphomimetic SynI mutants at sites 6,7 or site 7 only. The observation that the single site-7 mutant phenocopies the effects of the deletion of SynI identifies this site as the central switch in mediating the synaptic effects of Cdk5 and demonstrates that SynI is necessary and sufficient for achieving the effects of the kinase on SV trafficking. The phosphorylation state of SynI by Cdk5 at site 7 is regulated during chronic modification of neuronal activity and is an essential downstream effector for the Cdk5-mediated homeostatic scaling.
Keywords: Cdk5; actin; phosphorylation; synapsin; synaptic homeostasis; synaptic vesicle.
Copyright © 2014 the authors 0270-6474/14/347266-15$15.00/0.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
