SCUBE3 (signal peptide-CUB-EGF domain-containing protein 3) modulates fibroblast growth factor signaling during fast muscle development
- PMID: 24849601
- PMCID: PMC4081933
- DOI: 10.1074/jbc.M114.551929
SCUBE3 (signal peptide-CUB-EGF domain-containing protein 3) modulates fibroblast growth factor signaling during fast muscle development
Abstract
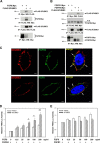
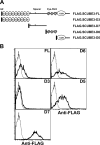
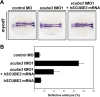
SCUBE3 (signal peptide CUB-EGF-like domain-containing protein 3) belongs to a newly identified secreted and cell membrane-associated SCUBE family, which is evolutionarily conserved in vertebrates. Scube3 is predominantly expressed in a variety of developing tissues in mice such as somites, neural tubes, and limb buds. However, its function during development remains unclear. In this study, we first showed that knockdown of SCUBE3 in C2C12 myoblasts inhibited FGF receptor 4 expression and FGF signaling, thus resulting in reduced myogenic differentiation. Furthermore, knockdown of zebrafish scube3 by antisense morpholino oligonucleotides specifically suppressed the expression of the myogenic marker myod1 within the lateral fast muscle precursors, whereas its expression in the adaxial slow muscle precursors was largely unaffected. Consistent with these findings, immunofluorescent staining of fast but not slow muscle myosin was markedly decreased in scube3 morphants. Further genetic studies identified fgf8 as a key regulator in scube3-mediated fast muscle differentiation in zebrafish. Biochemical and molecular analysis showed that SCUBE3 acts as a FGF co-receptor to augment FGF8 signaling. Scube3 may be a critical upstream regulator of fast fiber myogenesis by modulating fgf8 signaling during zebrafish embryogenesis.
Keywords: Cell Differentiation; Fast Muscle; Fibroblast Growth Factor (FGF); Fibroblast Growth Factor Receptor (FGFR); Myogenesis; Zebrafish; scube3.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
Zebrafish scube1 (signal peptide-CUB (complement protein C1r/C1s, Uegf, and Bmp1)-EGF (epidermal growth factor) domain-containing protein 1) is involved in primitive hematopoiesis.J Biol Chem. 2013 Feb 15;288(7):5017-26. doi: 10.1074/jbc.M112.375196. Epub 2012 Dec 27. J Biol Chem. 2013. PMID: 23271740 Free PMC article.
-
Fgf-driven Tbx protein activities directly induce myf5 and myod to initiate zebrafish myogenesis.Development. 2020 Apr 28;147(8):dev184689. doi: 10.1242/dev.184689. Development. 2020. PMID: 32345657 Free PMC article.
-
HtrA1 is a novel antagonist controlling fibroblast growth factor (FGF) signaling via cleavage of FGF8.Mol Cell Biol. 2012 Nov;32(21):4482-92. doi: 10.1128/MCB.00872-12. Epub 2012 Sep 4. Mol Cell Biol. 2012. PMID: 22949504 Free PMC article.
-
The biology of SCUBE.J Biomed Sci. 2023 May 26;30(1):33. doi: 10.1186/s12929-023-00925-3. J Biomed Sci. 2023. PMID: 37237303 Free PMC article. Review.
-
Numerous isoforms of Fgf8 reflect its multiple roles in the developing brain.J Cell Physiol. 2011 Jul;226(7):1722-6. doi: 10.1002/jcp.22587. J Cell Physiol. 2011. PMID: 21506104 Free PMC article. Review.
Cited by
-
Location, Location, Location: Signals in Muscle Specification.J Dev Biol. 2018 May 18;6(2):11. doi: 10.3390/jdb6020011. J Dev Biol. 2018. PMID: 29783715 Free PMC article. Review.
-
Unraveling the Mechanisms of Sensitivity to Anti-FGF Therapies in Imatinib-Resistant Gastrointestinal Stromal Tumors (GIST) Lacking Secondary KIT Mutations.Cancers (Basel). 2023 Nov 9;15(22):5354. doi: 10.3390/cancers15225354. Cancers (Basel). 2023. PMID: 38001614 Free PMC article.
-
"Muscling" Throughout Life: Integrating Studies of Muscle Development, Homeostasis, and Disease in Zebrafish.Curr Top Dev Biol. 2017;124:197-234. doi: 10.1016/bs.ctdb.2016.11.002. Epub 2016 Dec 23. Curr Top Dev Biol. 2017. PMID: 28335860 Free PMC article. Review.
-
Transcriptome Analysis Reveals Modulation of Human Stem Cells from the Apical Papilla by Species Associated with Dental Root Canal Infection.Int J Mol Sci. 2022 Nov 20;23(22):14420. doi: 10.3390/ijms232214420. Int J Mol Sci. 2022. PMID: 36430898 Free PMC article.
-
MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis.Int J Mol Sci. 2024 Apr 13;25(8):4308. doi: 10.3390/ijms25084308. Int J Mol Sci. 2024. PMID: 38673893 Free PMC article.
References
-
- Cheng C. J., Lin Y. C., Tsai M. T., Chen C. S., Hsieh M. C., Chen C. L., Yang R. B. (2009) SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 69, 3634–3641 - PubMed
-
- Grimmond S., Larder R., Van Hateren N., Siggers P., Hulsebos T. J., Arkell R., Greenfield A. (2000) Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1). Genomics 70, 74–81 - PubMed
-
- Haworth K., Smith F., Zoupa M., Seppala M., Sharpe P. T., Cobourne M. T. (2007) Expression of the Scube3 epidermal growth factor-related gene during early embryonic development in the mouse. Gene Expr. Patterns 7, 630–634 - PubMed
-
- Kawakami A., Nojima Y., Toyoda A., Takahoko M., Satoh M., Tanaka H., Wada H., Masai I., Terasaki H., Sakaki Y., Takeda H., Okamoto H. (2005) The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr. Biol. 15, 480–488 - PubMed
-
- Tsai M. T., Cheng C. J., Lin Y. C., Chen C. C., Wu A. R., Wu M. T., Hsu C. C., Yang R. B. (2009) Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem. J. 422, 119–128 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

