Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models
- PMID: 24849787
- PMCID: PMC4029740
- DOI: 10.1371/journal.pone.0097622
Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models
Abstract
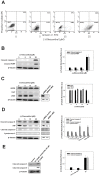
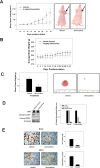
Dysregulation of the Hepatocyte growth factor (HGF)/c-Met signaling axis upregulates diverse tumor cell functions, including cell proliferation, survival, scattering and motility, epithelial-to-mesenchymal transition (EMT), angiogenesis, invasion, and metastasis. (-)-Oleocanthal is a naturally occurring secoiridoid from extra-virgin olive oil, which showed antiproliferative and antimigratory activity against different cancer cell lines. The aim of this study was to characterize the intracellular mechanisms involved in mediating the anticancer effects of (-)-oleocanthal treatment and the potential involvement of c-Met receptor signaling components in breast cancer. Results showed that (-)-oleocanthal inhibits the growth of human breast cancer cell lines MDA-MB-231, MCF-7 and BT-474 while similar treatment doses were found to have no effect on normal human MCF10A cell growth. In addition, (-)-oleocanthal treatment caused a dose-dependent inhibition of HGF-induced cell migration, invasion and G1/S cell cycle progression in breast cancer cell lines. Moreover, (-)-oleocanthal treatment effects were found to be mediated via inhibition of HGF-induced c-Met activation and its downstream mitogenic signaling pathways. This growth inhibitory effect is associated with blockade of EMT and reduction in cellular motility. Further results from in vivo studies showed that (-)-oleocanthal treatment suppressed tumor cell growth in an orthotopic model of breast cancer in athymic nude mice. Collectively, the findings of this study suggest that (-)-oleocanthal is a promising dietary supplement lead with potential for therapeutic use to control malignancies with aberrant c-Met activity.
Conflict of interest statement
Figures




References
-
- American Cancer Society, http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-ov... (Accessed November 2013).
-
- Giordano S, Ponzetto C, Di Renzo M, Cooper C, Comoglio P (1989) Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 11: 155–156. - PubMed
-
- Eder JP, Woude GFV, Boerner SA, LoRusso PM (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15: 2207–2214. - PubMed
-
- Peruzzi B, Bottaro DP (2006) Targeting the c-Met signaling pathway in cancer. Clin Cancer Res 12: 3657–3660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

