Policies and events affecting prescription opioid use for non-cancer pain among an insured patient population
- PMID: 24850102
- PMCID: PMC4716455
Policies and events affecting prescription opioid use for non-cancer pain among an insured patient population
Abstract
Background: Rising prescription opioid use and abuse have prompted widespread concern. However, to date there have been few rigorous investigations into the policies and events which may have contributed to these trends.
Objective: This study investigates trends in opioid use and related adverse events among individuals with non-cancer pain before and after implementation of major national policies.
Study design: The study used a longitudinal prospective study design. The analysis was limited to adults (age = 18 years) without a recorded cancer diagnosis. Pharmacy claims were used to assess rates of prescription opioid use, the strength of opioids dispensed, the proportion using opioids chronically, and related adverse events. Time trend analysis was used to identify changes in these rates over time. The study was Institutional Review Board approved.
Setting: Study patients were members of a large, health maintenance organization in southeast Michigan, with longitudinal records of prescription opioid use.
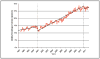
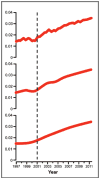
Results: The analysis comprised 523,623 individuals and 1,066,700 opioid pharmacy fills from January 1, 1997, to December 31, 2011. Contemporaneous with the implementation of health organization accreditation criteria requiring assessment and treatment of pain in all patients beginning January 2001, we observed a consistent and unabated increase in the rate of opioid fills and the proportion of chronic use. A parallel increase in the annual rate of adverse events was also observed. Similarly, we observed a continuous rise in the average strength of opioid fills following January 2001 with the exception of a single drop in December 2010, which was attributable to the withdrawal of propoxyphene from the U.S. market.
Limitations: This was an observational study and not a trial. Other long-term opioid-related benefits or harms, including functional status, quality of life, and substance use disorder, were not assessed.
Conclusions: This study provides temporal evidence for a rise in prescription opioid use after implementation of health organization accreditation criteria requiring standardized management of all individuals with pain.
Conflict of interest statement
Conflict of interest: Each author certifies that he or she, or a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript.
Figures





References
-
- Berry PH, Dahl JL. The new JCAHO pain standards: Implications for pain management nurses. Pain Manag Nurs. 2000;1:3–12. - PubMed
-
- Berry PH. Getting ready for JCAHO – just meeting the standards or really improving pain management. Clin J Oncol Nurs. 2001;5:110–112. - PubMed
-
- Lanser P, Gesell S. Pain management: The fifth vital sign. Healthc Benchmarks. 2001;8:68–70. 62. - PubMed
-
- Cleeland CS, Reyes-Gibby CC, Schall M, Nolan K, Paice J, Rosenberg JM, Tollett JH, Kerns RD. Rapid improvement in pain management: The Veterans Health Administration and the Institute for Healthcare improvement collaborative. Clin J Pain. 2003;19:298–305. - PubMed
-
- Phillips DM. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA. 2000;284:428–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
