Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases
- PMID: 24850149
- PMCID: PMC4086337
- DOI: 10.14348/molcells.2014.0104
Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases
Abstract
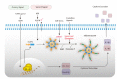
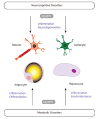
Inflammasomes are specialized signaling platforms critical for the regulation of innate immune and inflammatory responses. Various NLR family members (i.e., NLRP1, NLRP3, and IPAF) as well as the PYHIN family member AIM2 can form inflammasome complexes. These multi-protein complexes activate inflammatory caspases (i.e., caspase-1) which in turn catalyze the maturation of select pro-inflammatory cytokines, including interleukin (IL)-1β and IL-18. Activation of the NLRP3 inflammasome typically requires two initiating signals. Toll-like receptor (TLR) and NOD-like receptor (NLR) agonists activate the transcription of pro-inflammatory cytokine genes through an NF-κB-dependent priming signal. Following exposure to extracellular ATP, stimulation of the P2X purinoreceptor-7 (P2X7R), which results in K(+) efflux, is required as a second signal for NLRP3 inflammasome formation. Alternative models for NLRP3 activation involve lysosomal destabilization and phagocytic NADPH oxidase and/or mitochondria-dependent reactive oxygen species (ROS) production. In this review we examine regulatory mechanisms that activate the NLRP3 inflammasome pathway. Furthermore, we discuss the potential roles of NLRP3 in metabolic and cognitive diseases, including obesity, type 2 diabetes mellitus, Alzheimer's disease, and major depressive disorder. Novel therapeutics involving inflammasome activation may result in possible clinical applications in the near future.
Figures
References
-
- Alcocer-Gómez E., de Miguel M., Casas-Barquero N., Núñez-Vasco J., Sánchez-Alcazar J.A., Fernández-Rodríguez A., Cordero M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2013;36:111–117. - PubMed
-
- Balistreri C.R., Colonna-Romano G., Lio D., Candore G., Caruso C. TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. J. Clin. Immunol. 2009;29:406–415. - PubMed
-
- Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

