Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation
- PMID: 24850665
- PMCID: PMC4029171
- DOI: 10.7554/eLife.01817
Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation
Abstract
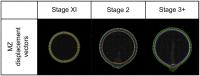
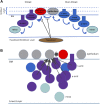
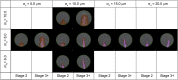
Gastrulation generates three layers of cells (ectoderm, mesoderm, endoderm) from a single sheet, while large scale cell movements occur across the entire embryo. In amniote (reptiles, birds, mammals) embryos, the deep layers arise by epithelial-to-mesenchymal transition (EMT) at a morphologically stable midline structure, the primitive streak (PS). We know very little about how these events are controlled or how the PS is maintained despite its continuously changing cellular composition. Using the chick, we show that isolated EMT events and ingression of individual cells start well before gastrulation. A Nodal-dependent 'community effect' then concentrates and amplifies EMT by positive feedback to form the PS as a zone of massive cell ingression. Computer simulations show that a combination of local cell interactions (EMT and cell intercalation) is sufficient to explain PS formation and the associated complex movements globally across a large epithelial sheet, without the need to invoke long-range signalling.DOI: http://dx.doi.org/10.7554/eLife.01817.001.
Keywords: EMT; cell movements; computer simulation; epithelial–mesenchymal interactions; modelling; primitive streak.
Copyright © 2014, Voiculescu et al.
Conflict of interest statement
LB: Lawrence Bodenstein is President and owner of Olana Technologies, Inc. which produced the computer program used for the simulations.
The other authors declare that no competing interests exist.
Figures






References
-
- Bachvarova RF, Skromne I, Stern CD. 1998. Induction of primitive streak and Hensen's node by the posterior marginal zone in the early chick embryo. Development 125:3521–3534 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

