Human immunodeficiency virus reverse transcriptase displays dramatically higher fidelity under physiological magnesium conditions in vitro
- PMID: 24850729
- PMCID: PMC4135932
- DOI: 10.1128/JVI.00752-14
Human immunodeficiency virus reverse transcriptase displays dramatically higher fidelity under physiological magnesium conditions in vitro
Erratum in
- J Virol. 2015 Feb;89(3):1944
Abstract
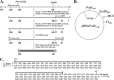
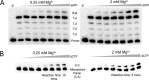
The fidelity of human immunodeficiency virus (HIV) reverse transcriptase (RT) has been a subject of intensive investigation. The mutation frequencies for the purified enzyme in vitro vary widely but are typically in the 10(-4) range (per nucleotide addition), making the enzyme severalfold less accurate than most polymerases, including other RTs. This has often been cited as a factor in HIV's accelerated generation of genetic diversity. However, cellular experiments suggest that HIV does not have significantly lower fidelity than other retroviruses and shows a mutation frequency in the 10(-5) range. In this report, we reconcile, at least in part, these discrepancies by showing that HIV RT fidelity in vitro is in the same range as cellular results from experiments conducted with physiological (for lymphocytes) concentrations of free Mg(2+) (~0.25 mM) and is comparable to Moloney murine leukemia virus (MuLV) RT fidelity. The physiological conditions produced mutation rates that were 5 to 10 times lower than those obtained under typically employed in vitro conditions optimized for RT activity (5 to 10 mM Mg(2+)). These results were consistent in both commonly used lacZα complementation and steady-state fidelity assays. Interestingly, although HIV RT showed severalfold-lower fidelity under high-Mg(2+) (6 mM) conditions, MuLV RT fidelity was insensitive to Mg(2+). Overall, the results indicate that the fidelity of HIV replication in cells is compatible with findings of experiments carried out in vitro with purified HIV RT, providing more physiological conditions are used.
Importance: Human immunodeficiency virus rapidly evolves through the generation and subsequent selection of mutants that can circumvent the immune response and escape drug therapy. This process is fueled, in part, by the presumably highly error-prone HIV polymerase reverse transcriptase (RT). Paradoxically, results of studies examining HIV replication in cells indicate an error frequency that is ~10 times lower than the rate for RT in the test tube, which invokes the possibility of factors that make RT more accurate in cells. This study brings the cellular and test tube results in closer agreement by showing that HIV RT is not more error prone than other RTs and, when assayed under physiological magnesium conditions, has a much lower error rate than in typical assays conducted using conditions optimized for enzyme activity.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
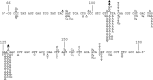


References
-
- Telesnitsky A, Goff SP. 1993. Reverse transcriptase. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY - PubMed
-
- Cowan JA, Ohyama T, Howard K, Rausch JW, Cowan SM, Le Grice SF. 2000. Metal-ion stoichiometry of the HIV-1 RT ribonuclease H domain: evidence for two mutually exclusive sites leads to new mechanistic insights on metal-mediated hydrolysis in nucleic acid biochemistry. J. Biol. Inorg. Chem. 5:67–74. 10.1007/s007750050009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

