MDA5 and LGP2: accomplices and antagonists of antiviral signal transduction
- PMID: 24850739
- PMCID: PMC4135949
- DOI: 10.1128/JVI.00640-14
MDA5 and LGP2: accomplices and antagonists of antiviral signal transduction
Abstract
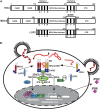
Mammalian cells have the ability to recognize virus infection and mount a powerful antiviral transcriptional response that provides an initial barrier to replication and impacts both innate and adaptive immune responses. Retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) proteins mediate intracellular virus recognition and are activated by viral RNA ligands to induce antiviral signal transduction. While the mechanisms of RIG-I regulation are already well understood, less is known about the more enigmatic melanoma differentiation-associated 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2). Emerging evidence suggests that these two RLRs are intimately associated as both accomplices and antagonists of antiviral signal transduction.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Sun YW. 1997. RIG-I, a human homolog gene of RNA helicase, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. Ph.D. dissertation Shanghai Second Medical University, Shanghai, China
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

