Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line
- PMID: 24850911
- PMCID: PMC4042850
- DOI: 10.1098/rsob.140041
Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line
Abstract
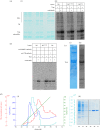
Striated muscle tissues undergo adaptive remodelling in response to mechanical load. This process involves the myofilament titin and, specifically, its kinase domain (TK; titin kinase) that translates mechanical signals into regulatory pathways of gene expression in the myofibril. TK mechanosensing appears mediated by a C-terminal regulatory tail (CRD) that sterically inhibits its active site. Allegedly, stretch-induced unfolding of this tail during muscle function releases TK inhibition and leads to its catalytic activation. However, the cellular pathway of TK is poorly understood and substrates proposed to date remain controversial. TK's best-established substrate is Tcap, a small structural protein of the Z-disc believed to link TK to myofibrillogenesis. Here, we show that TK is a pseudokinase with undetectable levels of catalysis and, therefore, that Tcap is not its substrate. Inactivity is the result of two atypical residues in TK's active site, M34 and E147, that do not appear compatible with canonical kinase patterns. While not mediating stretch-dependent phospho-transfers, TK binds the E3 ubiquitin ligase MuRF1 that promotes sarcomeric ubiquitination in a stress-induced manner. Given previous evidence of MuRF2 interaction, we propose that the cellular role of TK is to act as a conformationally regulated scaffold that functionally couples the ubiquitin ligases MuRF1 and MuRF2, thereby coordinating muscle-specific ubiquitination pathways and myofibril trophicity. Finally, we suggest that an evolutionary dichotomy of kinases/pseudokinases has occurred in TK-like kinases, where invertebrate members are active enzymes but vertebrate counterparts perform their signalling function as pseudokinase scaffolds.
Keywords: mutagenesis; phosphorylation assay; pseudokinase; titin.
Figures




References
-
- Granzier HL, Labeit S. 2005. Titin and its associated proteins: the third myofilament system of the sarcomere. Adv. Protein Chem. 71, 89–119. (doi:10.1016/S0065-3233(04)71003-7) - DOI - PubMed
-
- Knoll R, et al. 2011. Telethonin deficiency is associated with maladaptation to biomechanical stress in the mammalian heart. Circ. Res. 109, 758–769. (doi:10.1161/CIRCRESAHA.111.245787) - DOI - PMC - PubMed
-
- Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. 2002. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J. Cell Sci. 115, 4925–4936. (doi:10.1242/jcs.00181) - DOI - PubMed
-
- Miller MK, et al. 2003. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J. Mol. Biol. 333, 951–964. (doi:10.1016/j.jmb.2003.09.012) - DOI - PubMed
-
- Ojima K, et al. 2010. Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J. Clin. Invest. 120, 2672–2683. (doi:10.1172/JCI40658) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

