Meta-analysis of first-line triple therapy for helicobacter pylori eradication in Korea: is it time to change?
- PMID: 24851029
- PMCID: PMC4024949
- DOI: 10.3346/jkms.2014.29.5.704
Meta-analysis of first-line triple therapy for helicobacter pylori eradication in Korea: is it time to change?
Abstract
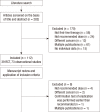
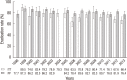
Proton pump inhibitor (PPI)-based triple therapy consisting of PPI, amoxicillin, and clarithromycin, is the recommended first-line treatment for Helicobacter pylori infection. However, the eradication rate of triple therapy has declined over the past few decades. We analyzed the eradication rate and adverse events of triple therapy to evaluate current practices in Korea. A comprehensive literature search was performed up to August 2013 of 104 relevant studies comprising 42,124 patients. The overall eradication rate was 74.6% (95% confidence interval [CI], 72.1%-77.2%) by intention-to-treat analysis and 82.0% (95% CI, 80.8%-83.2%) by per-protocol analysis. The eradication rate decreased significantly from 1998 to 2013 (P < 0.001 for both intention-to-treat and per-protocol analyses). Adverse events were reported in 41 studies with 8,018 subjects with an overall incidence rate of 20.4% (95% CI, 19.6%-21.3%). The available data suggest that the effectiveness of standard triple therapy for H. pylori eradication has decreased to an unacceptable level. A novel therapeutic strategy is warranted to improve the effectiveness of first-line treatment for H. pylori infection in Korea.
Keywords: Eradication; Helicobacter pylori; Triple Therapy.
Conflict of interest statement
The authors have no competing conflicts of interest to disclose.
Figures
Similar articles
-
Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies.World J Gastroenterol. 2015 Jan 7;21(1):351-9. doi: 10.3748/wjg.v21.i1.351. World J Gastroenterol. 2015. PMID: 25574111 Free PMC article. Clinical Trial.
-
A comparison between 15-day sequential, 10-day sequential and proton pump inhibitor-based triple therapy for Helicobacter pylori infection in Korea.Scand J Gastroenterol. 2014 Aug;49(8):917-24. doi: 10.3109/00365521.2014.896409. Epub 2014 Jul 3. Scand J Gastroenterol. 2014. PMID: 24988873 Clinical Trial.
-
Treatment options for Helicobacter pylori infection when proton pump inhibitor-based triple therapy fails in clinical practice.Aliment Pharmacol Ther. 1999 Apr;13(4):489-96. doi: 10.1046/j.1365-2036.1999.00504.x. Aliment Pharmacol Ther. 1999. PMID: 10215733 Clinical Trial.
-
[Changes in the eradication rate of conventional triple therapy for Helicobacter pylori infection in Korea].Korean J Gastroenterol. 2014 Mar 25;63(3):141-5. doi: 10.4166/kjg.2014.63.3.141. Korean J Gastroenterol. 2014. PMID: 24651586 Review. Korean.
-
Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough.Aliment Pharmacol Ther. 2011 Dec;34(11-12):1255-68. doi: 10.1111/j.1365-2036.2011.04887.x. Epub 2011 Oct 21. Aliment Pharmacol Ther. 2011. PMID: 22017749 Review.
Cited by
-
Comparison of Helicobacter pylori Eradication Rates Using Standard Triple Therapy and Sequential Therapy.Korean J Helicobacter Up Gastrointest Res. 2023 Dec;23(4):277-282. doi: 10.7704/kjhugr.2023.0042. Epub 2023 Dec 8. Korean J Helicobacter Up Gastrointest Res. 2023. PMID: 40503496 Free PMC article.
-
[Can Sequential Therapy Replace Standard Triple Therapy as Helicobacter pylori Eradication?].Korean J Helicobacter Up Gastrointest Res. 2023 Dec;23(4):235-236. doi: 10.7704/kjhugr.2023.0047. Epub 2023 Dec 8. Korean J Helicobacter Up Gastrointest Res. 2023. PMID: 40503503 Free PMC article. Korean. No abstract available.
-
Appropriate first-line regimens to combat Helicobacter pylori antibiotic resistance: an Asian perspective.Molecules. 2015 Apr 8;20(4):6068-92. doi: 10.3390/molecules20046068. Molecules. 2015. PMID: 25856059 Free PMC article. Review.
-
Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea.Gut Liver. 2019 Sep 15;13(5):531-540. doi: 10.5009/gnl19136. Gut Liver. 2019. PMID: 31505907 Free PMC article. Clinical Trial.
-
Optimal First-Line Treatment for Helicobacter pylori Infection: Recent Strategies.Gastroenterol Res Pract. 2016;2016:9086581. doi: 10.1155/2016/9086581. Epub 2016 Dec 13. Gastroenterol Res Pract. 2016. PMID: 28070184 Free PMC article. Review.
References
-
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. - PubMed
-
- McColl KE. Clinical practice: helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. - PubMed
-
- Jung JH, Choi KD, Han S, Jung HY, Do MY, Chang HS, Choe JW, Lee GH, Song HJ, Kim DH, et al. Seroconversion rates of Helicobacter pylori infection in Korean adults. Helicobacter. 2013;18:299–308. - PubMed
-
- Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

