Preparation of biocompatible heat-labile enterotoxin subunit B-bovine serum albumin nanoparticles for improving tumor-targeted drug delivery via heat-labile enterotoxin subunit B mediation
- PMID: 24851048
- PMCID: PMC4018319
- DOI: 10.2147/IJN.S60764
Preparation of biocompatible heat-labile enterotoxin subunit B-bovine serum albumin nanoparticles for improving tumor-targeted drug delivery via heat-labile enterotoxin subunit B mediation
Abstract
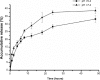
Heat-labile enterotoxin subunit B (LTB) is a non-catalytic protein from a pentameric subunit of Escherichia coli. Based on its function of binding specifically to ganglioside GM1 on the surface of cells, a novel nanoparticle (NP) composed of a mixture of bovine serum albumin (BSA) and LTB was designed for targeted delivery of 5-fluorouracil to tumor cells. BSA-LTB NPs were characterized by determination of their particle size, polydispersity, morphology, drug encapsulation efficiency, and drug release behavior in vitro. The internalization of fluorescein isothiocyanate-labeled BSA-LTB NPs into cells was observed using fluorescent imaging. Results showed that BSA-LTB NPs presented a narrow size distribution with an average hydrodynamic diameter of approximately 254±19 nm and a mean zeta potential of approximately -19.95±0.94 mV. In addition, approximately 80.1% of drug was encapsulated in NPs and released in the biphasic pattern. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that BSA-LTB NPs exhibited higher cytotoxic activity than non-targeted NPs (BSA NPs) in SMMC-7721 cells. Fluorescent imaging results proved that, compared with BSA NPs, BSA-LTB NPs could greatly enhance cellular uptake. Hence, the results indicate that BSA-LTB NPs could be a potential nanocarrier to improve targeted delivery of 5-fluorouracil to tumor cells via mediation of LTB.
Keywords: 5-fluorouracil; bovine serum albumin; heat-labile enterotoxin subunit B; nanoparticle.
Figures




References
-
- Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64(2):327–336. - PubMed
-
- Jensen AB, Hansen O, Jørgensen K, Bastholt L. Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol. 1994;33(5):487–491. - PubMed
-
- Marijnen CA, Kapiteijn E, van de Velde CJ, et al. Cooperative Investigators of the Dutch Colorectal Cancer Group Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20(3):817–825. - PubMed
-
- Zachariah B, Balducci L, Venkattaramanabalaji GV, Casey L, Greenberg HM, DelRegato JA. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39(5):1125–1129. - PubMed
-
- Chen H, Yang W, Chen H, et al. Surface modification of mitoxantrone-loaded PLGA nanospheres with chitosan. Colloids Surf B Biointerfaces. 2009;73(2):212–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

