The effectiveness of dimethyl fumarate monotherapy in the treatment of relapsing-remitting multiple sclerosis: a systematic review and meta-analysis
- PMID: 24851089
- PMCID: PMC4023455
- DOI: 10.2174/1570159X12666140115214801
The effectiveness of dimethyl fumarate monotherapy in the treatment of relapsing-remitting multiple sclerosis: a systematic review and meta-analysis
Abstract
Background: Dimethyl fumarate (BG-12, Tecfidera®) is a new oral drug approved by FDA and EMA in March 2013 for relapsing - remitting multiple sclerosis (RRMS). The drug was much anticipated because of its possible superiority over currently available medications: fingolimod and teriflunomide as the only MS treatments currently available in oral form.
Objective: The aim of this systematic review with meta-analysis was to assess the efficacy and safety of BG-12 in the treatment of RRMS.
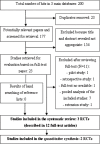
Methods: A systematic literature search was conducted in Medline/PubMed, EMBASE, and Cochrane Library up till 3(rd) November, 2013. We sought all published randomized clinical trials evaluating the use of dimethyl fumarate for the treatment of patients with RRMS. All included studies were critically appraised and analyzed with the use of Review Manager 5.1.0. software according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol.
Results: Two trials, DEFINE and CONFIRM involved 2 651 patients and compared dimethyl fumarate taken either two or three times daily with placebo in patients with RRMS. Additionally in CONFIRM trial third group of patients received glatiramer acetate. The overall results of the meta-analysis showed that BG-12 (at both dosages) given to patients with RRMS is safe and statistically significantly more effective than placebo in reducing the proportion of patients who had a relapse by 2 years, the rate of disability progression and the mean number of gadolinium-enhancing lesions at 2 years. The comparison between BG-12 and glatiramer acetate revealed that the analyzed agent could potentially be more effective in the treatment of RRMS.
Conclusions: Despite limited RCTs data available, both analyzed BG-12 regimens showed their efficacy on clinical disease parameters and other measures of disease activity in RRMS. The safety profile of the study agent was acceptable.
Keywords: Autoimmune disorder; dimethyl fumarate; disease-modifying therapies; relapsing-remitting multiple sclerosis..
Figures



References
-
- Limmroth V. Multiple sclerosis: oral BG12 for treatment of relapsing-remitting MS. Nat. Rev. Neurol. 2013;9:8–10. - PubMed
-
- Tullman M. A Review of current and emerging therapeutic strategies in multiple sclerosis. Am. J. Manag Care. 2013;19:21–27. - PubMed
-
- Fox EJ, Rhoades RW. New treatments and treatment goals for patients with relapsing-remitting multiple sclerosis. Curr. Opin. Neurol. 2012;25 Suppl:11–19. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
