Diagnostic Value of Circulating Extracellular miR-134, miR-185, and miR-22 Levels in Lung Adenocarcinoma-Associated Malignant Pleural Effusion
- PMID: 24851110
- PMCID: PMC4022827
- DOI: 10.4143/crt.2014.46.2.178
Diagnostic Value of Circulating Extracellular miR-134, miR-185, and miR-22 Levels in Lung Adenocarcinoma-Associated Malignant Pleural Effusion
Abstract
Purpose: The accurate and timely diagnosis of malignant pleural effusion (MPE) in lung cancer patients is important because MPE has a poor prognosis and is classified as stage IV disease. Molecular biomarkers for pleural effusion, such as circulating extracellular microRNAs (miRNAs) isolated from pleural fluid, may help in the diagnosis of MPE. The present study examined whether miRNAs that are deregulated in lung cancer (miR-134, miR-185, and miR-22) can serve as diagnostic markers for lung adenocarcinoma-associated MPE (LA-MPE).
Materials and methods: Real-time reverse transcription quantitative polymerase chain reaction was used to measure the expression of the three miRNAs in samples from 87 patients with pleural effusion comprising 45 LA-MPEs and 42 benign pleural effusions (BPEs). The area under the receiver operating characteristic curve (AUC) was then used to evaluate the diagnostic performance of each of the three miRNAs and compare it with that of the common tumor marker, carcinoembryonic antigen (CEA).
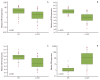
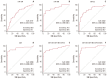
Results: The expression of all three miRNAs was significantly lower in LA-MPE than in BPE (p <0.001). The AUCs for miR-134, miR-185, miR-22, and CEA were 0.721, 0.882, 0.832, and 0.898, respectively. Combining CEA with the three miRNAs increased the diagnostic performance, yielding an AUC of 0.942 (95% confidence interval, 0.864 to 0.982), with a sensitivity of 91.9% and a specificity of 92.5%.
Conclusion: The present study suggests that the expression levels of circulating extracellular miR-134, miR-185, and miR-22 in patients with pleural effusion may have diagnostic value when differentiating between LA-MPE and BPE.
Keywords: Adenocarcinoma; Lung; Pleural effusion; miR-134; miR-185; miR-22.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
References
-
- Memon A, Zawadzki ZA. Malignant effusions: diagnostic evaluation and therapeutic strategy. Curr Probl Cancer. 1981;5:1–30. - PubMed
-
- Johnston WW. The malignant pleural effusion: a review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905–909. - PubMed
-
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. - PubMed
-
- See KC, Lee P. Advances in the diagnosis of pleural disease in lung cancer. Ther Adv Respir Dis. 2011;5:409–418. - PubMed
-
- Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320–324. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

