The cerebellum and addiction: insights gained from neuroimaging research
- PMID: 24851284
- PMCID: PMC4031616
- DOI: 10.1111/adb.12101
The cerebellum and addiction: insights gained from neuroimaging research
Abstract
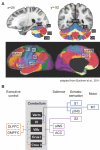
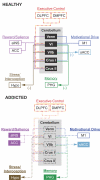
Although cerebellar alterations have been consistently noted in the addiction literature, the pathophysiology of this link remains unclear. The cerebellum is commonly classified as a motor structure, but human functional neuroimaging along with clinical observations in cerebellar stroke patients and anatomical tract tracing in non-human primates suggests its involvement in cognitive and affective processing. A comprehensive literature search on the role of the cerebellum in addiction was performed. This review article (1) considers the potential role of the cerebellum in addiction; (2) summarizes the cerebellar structural alterations linked to addiction; (3) presents the functional neuroimaging evidence linking the cerebellum with addiction; and (4) proposes a model for addiction that underscores the role of the cerebellum. The data implicate the cerebellum as an intermediary between motor and reward, motivation and cognitive control systems, as all are relevant etiologic factors in addiction. Furthermore, consideration of these findings could contribute to deeper and more sophisticated insights into normal reward and motivational function. The goal of this review is to spread awareness of cerebellar involvement in addictive processes, and to suggest a preliminary model for its potential role.
Figures


References
-
- Anderson CM, Maas LC, Frederick B. deB, Bendor JT, Spencer TJ, Livni E, Lukas ES, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31(6):1318–1326. - PubMed
-
- Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cannabinoid CB1 receptor in inhibitory interneurons in the cerebellum. Cerebellum. 2004;3(4):222–226. - PubMed
-
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. NeuroImage. 2011;56(3):1021–1026. - PubMed
-
- Bauer DJ, Kerr AL, Swain RA. Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration. Neurobiology of learning and memory. 2011;95(2):166–175. - PubMed
-
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction biology. 2007;12(1):122–132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

