An innovative influenza vaccination policy: targeting last season's patients
- PMID: 24851863
- PMCID: PMC4031061
- DOI: 10.1371/journal.pcbi.1003643
An innovative influenza vaccination policy: targeting last season's patients
Abstract
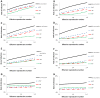
Influenza vaccination is the primary approach to prevent influenza annually. WHO/CDC recommendations prioritize vaccinations mainly on the basis of age and co-morbidities, but have never considered influenza infection history of individuals for vaccination targeting. We evaluated such influenza vaccination policies through small-world contact networks simulations. Further, to verify our findings we analyzed, independently, large-scale empirical data of influenza diagnosis from the two largest Health Maintenance Organizations in Israel, together covering more than 74% of the Israeli population. These longitudinal individual-level data include about nine million cases of influenza diagnosed over a decade. Through contact network epidemiology simulations, we found that individuals previously infected with influenza have a disproportionate probability of being highly connected within networks and transmitting to others. Therefore, we showed that prioritizing those previously infected for vaccination would be more effective than a random vaccination policy in reducing infection. The effectiveness of such a policy is robust over a range of epidemiological assumptions, including cross-reactivity between influenza strains conferring partial protection as high as 55%. Empirically, our analysis of the medical records confirms that in every age group, case definition for influenza, clinical diagnosis, and year tested, patients infected in the year prior had a substantially higher risk of becoming infected in the subsequent year. Accordingly, considering individual infection history in targeting and promoting influenza vaccination is predicted to be a highly effective supplement to the current policy. Our approach can also be generalized for other infectious disease, computer viruses, or ecological networks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Fiore AE, Uyeki TM, Broder K, Finelli GL, Singleton J, et al. (2010) Prevention and Control of Influenza with Vaccines. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5908a1.htm. Accessed 30 October 2012. - PubMed
-
- Stephenson I, Hayden F, Osterhaus A, Howard W, Pervikov Y, et al. (2010) Report of the fourth meeting on “Influenza vaccines that induce broad spectrum and long-lasting immune responses”, World Health Organization and Wellcome Trust, London, United Kingdom, 9–10 November 2009. Vaccine 28: 3875–3882 Available: http://www.ncbi.nlm.nih.gov/pubmed/20398616. Accessed 18 November 2012. - PubMed
-
- Balicer RD, Huerta M, Davidovitch N, Grotto I (2005) Cost-benefit of stockpiling drugs for influenza pandemic. Emerg Infect Dis 11: 1280–1282 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3320484&tool=p.... Accessed 2 January 2014. - PMC - PubMed
-
- WHO | Influenza (2003). World Heal Organ. Available: http://www.who.int/mediacentre/factsheets/2003/fs211/en/. Accessed 1 January 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

