Inactivation of fructose-1,6-bisphosphate aldolase prevents optimal co-catabolism of glycolytic and gluconeogenic carbon substrates in Mycobacterium tuberculosis
- PMID: 24851864
- PMCID: PMC4031216
- DOI: 10.1371/journal.ppat.1004144
Inactivation of fructose-1,6-bisphosphate aldolase prevents optimal co-catabolism of glycolytic and gluconeogenic carbon substrates in Mycobacterium tuberculosis
Abstract
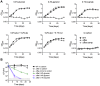
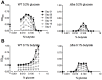
Metabolic pathways used by Mycobacterium tuberculosis (Mtb) to establish and maintain infections are important for our understanding of pathogenesis and the development of new chemotherapies. To investigate the role of fructose-1,6-bisphosphate aldolase (FBA), we engineered an Mtb strain in which FBA levels were regulated by anhydrotetracycline. Depletion of FBA resulted in clearance of Mtb in both the acute and chronic phases of infection in vivo, and loss of viability in vitro when cultured on single carbon sources. Consistent with prior reports of Mtb's ability to co-catabolize multiple carbon sources, this in vitro essentiality could be overcome when cultured on mixtures of glycolytic and gluconeogenic carbon sources, enabling generation of an fba knockout (Δfba). In vitro studies of Δfba however revealed that lack of FBA could only be compensated for by a specific balance of glucose and butyrate in which growth and metabolism of butyrate were determined by Mtb's ability to co-catabolize glucose. These data thus not only evaluate FBA as a potential drug target in both replicating and persistent Mtb, but also expand our understanding of the multiplicity of in vitro conditions that define the essentiality of Mtb's FBA in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Glycolytic and non-glycolytic functions of Mycobacterium tuberculosis fructose-1,6-bisphosphate aldolase, an essential enzyme produced by replicating and non-replicating bacilli.J Biol Chem. 2011 Nov 18;286(46):40219-31. doi: 10.1074/jbc.M111.259440. Epub 2011 Sep 23. J Biol Chem. 2011. PMID: 21949126 Free PMC article.
-
The metabolic enzyme fructose-1,6-bisphosphate aldolase acts as a transcriptional regulator in pathogenic Francisella.Nat Commun. 2017 Oct 11;8(1):853. doi: 10.1038/s41467-017-00889-7. Nat Commun. 2017. PMID: 29021545 Free PMC article.
-
Metabolic adaptation of two in silico mutants of Mycobacterium tuberculosis during infection.BMC Syst Biol. 2017 Nov 21;11(1):107. doi: 10.1186/s12918-017-0496-z. BMC Syst Biol. 2017. PMID: 29157227 Free PMC article.
-
Fructose-1,6-bisphosphate aldolase (FBA)-a conserved glycolytic enzyme with virulence functions in bacteria: 'ill met by moonlight'.Biochem Soc Trans. 2014 Dec;42(6):1792-5. doi: 10.1042/BST20140203. Biochem Soc Trans. 2014. PMID: 25399608 Review.
-
Fructose 1,6-bisphosphate aldolase: A promising prognostic marker for oral cancer and its role in radiotherapy response.Radiother Oncol. 2024 Nov;200:110537. doi: 10.1016/j.radonc.2024.110537. Epub 2024 Sep 13. Radiother Oncol. 2024. PMID: 39278318 Review.
Cited by
-
CitE Enzymes Are Essential for Mycobacterium tuberculosis to Establish Infection in Macrophages and Guinea Pigs.Front Cell Infect Microbiol. 2018 Nov 6;8:385. doi: 10.3389/fcimb.2018.00385. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30460206 Free PMC article.
-
Derailing the aspartate pathway of Mycobacterium tuberculosis to eradicate persistent infection.Nat Commun. 2019 Sep 16;10(1):4215. doi: 10.1038/s41467-019-12224-3. Nat Commun. 2019. PMID: 31527595 Free PMC article.
-
Importance of Metabolic Adaptations in Francisella Pathogenesis.Front Cell Infect Microbiol. 2017 Mar 28;7:96. doi: 10.3389/fcimb.2017.00096. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28401066 Free PMC article. Review.
-
Depleting Mycobacterium tuberculosis of the transcription termination factor Rho causes pervasive transcription and rapid death.Nat Commun. 2017 Mar 28;8:14731. doi: 10.1038/ncomms14731. Nat Commun. 2017. PMID: 28348398 Free PMC article.
-
The FBPase Encoding Gene glpX Is Required for Gluconeogenesis, Bacterial Proliferation and Division In Vivo of Mycobacterium marinum.PLoS One. 2016 May 27;11(5):e0156663. doi: 10.1371/journal.pone.0156663. eCollection 2016. PLoS One. 2016. PMID: 27233038 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

