Novel epigenetic target therapy for prostate cancer: a preclinical study
- PMID: 24851905
- PMCID: PMC4031137
- DOI: 10.1371/journal.pone.0098101
Novel epigenetic target therapy for prostate cancer: a preclinical study
Abstract
Epigenetic events are critical contributors to the pathogenesis of cancer, and targeting epigenetic mechanisms represents a novel strategy in anticancer therapy. Classic demethylating agents, such as 5-Aza-2'-deoxycytidine (Decitabine), hold the potential for reprograming somatic cancer cells demonstrating high therapeutic efficacy in haematological malignancies. On the other hand, epigenetic treatment of solid tumours often gives rise to undesired cytotoxic side effects. Appropriate delivery systems able to enrich Decitabine at the site of action and improve its bioavailability would reduce the incidence of toxicity on healthy tissues. In this work we provide preclinical evidences of a safe, versatile and efficient targeted epigenetic therapy to treat hormone sensitive (LNCap) and hormone refractory (DU145) prostate cancers. A novel Decitabine formulation, based on the use of engineered erythrocyte (Erythro-Magneto-Hemagglutinin Virosomes, EMHVs) drug delivery system (DDS) carrying this drug, has been refined. Inside the EMHVs, the drug was shielded from the environment and phosphorylated in its active form. The novel magnetic EMHV DDS, endowed with fusogenic protein, improved the stability of the carried drug and exhibited a high efficiency in confining its delivery at the site of action in vivo by applying an external static magnetic field. Here we show that Decitabine loaded into EMHVs induces a significant tumour mass reduction in prostate cancer xenograft models at a concentration, which is seven hundred times lower than the therapeutic dose, suggesting an improved pharmacokinetics/pharmacodynamics of drug. These results are relevant for and discussed in light of developing personalised autologous therapies and innovative clinical approach for the treatment of solid tumours.
Conflict of interest statement
Figures
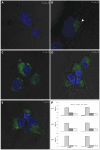


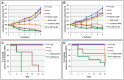

References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2008) Cancer statistics, 2008. CA Cancer J Clin 58: 71–96. - PubMed
-
- Scosyrev E, Messing EM, Mohile S, Golijanin D, Wu G (2012) Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer 118: 3062–3070. - PubMed
-
- Gnanapragasam VJ, Mason MD, Shaw GL, Neal DE (2012) The role of surgery in high-risk localised prostate cancer. BJU Int 109: 648–658. - PubMed
-
- Shipley WU, Thames HD, Sandler HM, Hanks GE, Zietman AL, et al. (1999) Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA 281: 1598–1604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

