Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7
- PMID: 24851944
- PMCID: PMC4031124
- DOI: 10.1371/journal.pntd.0002885
Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7
Abstract
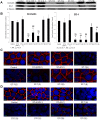
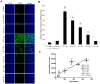
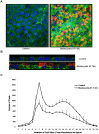
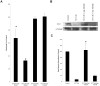
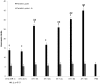
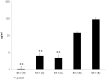
Blastocystis is an extracellular, enteric pathogen that induces intestinal disorders in a range of hosts including humans. Recent studies have identified potential parasite virulence factors in and host responses to this parasite; however, little is known about Blastocystis-host attachment, which is crucial for colonization and virulence of luminal stages. By utilizing 7 different strains of the parasite belonging to two clinically relevant subtypes ST-4 and ST-7, we investigated Blastocystis-enterocyte adhesion and its association with parasite-induced epithelial barrier disruption. We also suggest that drug resistance in ST-7 strains might result in fitness cost that manifested as impairment of parasite adhesion and, consequently, virulence. ST-7 parasites were generally highly adhesive to Caco-2 cells and preferred binding to intercellular junctions. These strains also induced disruption of ZO-1 and occludin tight junction proteins as well as increased dextran-FITC flux across epithelial monolayers. Interestingly, their adhesion was correlated with metronidazole (Mz) susceptibility. Mz resistant (Mzr) strains were found to be less pathogenic, owing to compromised adhesion. Moreover, tolerance of nitrosative stress was also reduced in the Mzr strains. In conclusion, the findings indicate that Blastocystis attaches to intestinal epithelium and leads to epithelial barrier dysfunction and that drug resistance might entail a fitness cost in parasite virulence by limiting entero-adhesiveness. This is the first study of the cellular basis for strain-to-strain variation in parasite pathogenicity. Intra- and inter-subtype variability in cytopathogenicity provides a possible explanation for the diverse clinical outcomes of Blastocystis infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Scanlan PD (2012) Blastocystis: past pitfalls and future perspectives. Trends Parasitol 28: 327–334. - PubMed
-
- Tan KS, Mirza H, Teo JD, Wu B, Macary PA (2010) Current Views on the Clinical Relevance of Blastocystis spp. Curr Infect Dis Rep 12: 28–35. - PubMed
-
- Alfellani MA, Taner-Mulla D, Jacob AS, Imeede CA, Yoshikawa H, et al. (2013) Genetic diversity of blastocystis in livestock and zoo animals. Protist 164: 497–509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

