BCA2/Rabring7 targets HIV-1 Gag for lysosomal degradation in a tetherin-independent manner
- PMID: 24852021
- PMCID: PMC4031200
- DOI: 10.1371/journal.ppat.1004151
BCA2/Rabring7 targets HIV-1 Gag for lysosomal degradation in a tetherin-independent manner
Abstract
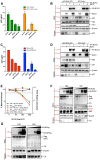
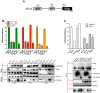
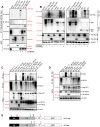
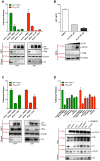
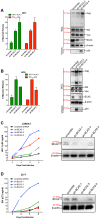
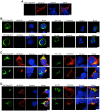
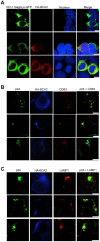
BCA2 (Rabring7, RNF115 or ZNF364) is a RING-finger E3 ubiquitin ligase that was identified as a co-factor in the restriction imposed by tetherin/BST2 on HIV-1. Contrary to the current model, in which BCA2 lacks antiviral activity in the absence of tetherin, we found that BCA2 possesses tetherin-independent antiviral activity. Here we show that the N-terminus of BCA2 physically interacts with the Matrix region of HIV-1 and other retroviral Gag proteins and promotes their ubiquitination, redistribution to endo-lysosomal compartments and, ultimately, lysosomal degradation. The targeted depletion of BCA2 in tetherin-expressing and tetherin-deficient cells results in a significant increase in virus release and replication, indicating that endogenous BCA2 possesses antiviral activity. Therefore, these results indicate that BCA2 functions as an antiviral factor that targets HIV-1 Gag for degradation, impairing virus assembly and release.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
BCA2/Rabring7 promotes tetherin-dependent HIV-1 restriction.PLoS Pathog. 2009 Dec;5(12):e1000700. doi: 10.1371/journal.ppat.1000700. Epub 2009 Dec 18. PLoS Pathog. 2009. PMID: 20019814 Free PMC article.
-
Antiviral Activity of Feline BCA2 Is Mainly Dependent on Its Interference With Proviral Transcription Rather Than Degradation of FIV Gag.Front Microbiol. 2020 Jun 11;11:1230. doi: 10.3389/fmicb.2020.01230. eCollection 2020. Front Microbiol. 2020. PMID: 32595622 Free PMC article.
-
BCA2/Rabring7 Interferes with HIV-1 Proviral Transcription by Enhancing the SUMOylation of IκBα.J Virol. 2017 Mar 29;91(8):e02098-16. doi: 10.1128/JVI.02098-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28122985 Free PMC article.
-
Multifaceted Roles of the E3 Ubiquitin Ligase RING Finger Protein 115 in Immunity and Diseases.Front Immunol. 2022 Jun 30;13:936579. doi: 10.3389/fimmu.2022.936579. eCollection 2022. Front Immunol. 2022. PMID: 35844553 Free PMC article. Review.
-
Tetherin and its viral antagonists.J Neuroimmune Pharmacol. 2011 Jun;6(2):188-201. doi: 10.1007/s11481-010-9256-1. Epub 2011 Jan 11. J Neuroimmune Pharmacol. 2011. PMID: 21222046 Free PMC article. Review.
Cited by
-
Broad-Spectrum Antiviral Activity of an Ankyrin Repeat Protein on Viral Assembly against Chimeric NL4-3 Viruses Carrying Gag/PR Derived from Circulating Strains among Northern Thai Patients.Viruses. 2018 Nov 13;10(11):625. doi: 10.3390/v10110625. Viruses. 2018. PMID: 30428529 Free PMC article.
-
The E3 ubiquitin ligase RNF115 regulates phagosome maturation and host response to bacterial infection.EMBO J. 2022 Dec 1;41(23):e108970. doi: 10.15252/embj.2021108970. Epub 2022 Oct 25. EMBO J. 2022. PMID: 36281581 Free PMC article.
-
MCPIP1/regnase-I inhibits simian immunodeficiency virus and is not counteracted by Vpx.J Gen Virol. 2016 Jul;97(7):1693-1698. doi: 10.1099/jgv.0.000482. Epub 2016 Apr 13. J Gen Virol. 2016. PMID: 27075251 Free PMC article.
-
ATP1B3 Protein Modulates the Restriction of HIV-1 Production and Nuclear Factor κ Light Chain Enhancer of Activated B Cells (NF-κB) Activation by BST-2.J Biol Chem. 2016 Feb 26;291(9):4754-62. doi: 10.1074/jbc.M115.679357. Epub 2015 Dec 22. J Biol Chem. 2016. PMID: 26694617 Free PMC article.
-
Disulfiram ameliorates STING/MITA-dependent inflammation and autoimmunity by targeting RNF115.Cell Mol Immunol. 2024 Mar;21(3):275-291. doi: 10.1038/s41423-024-01131-3. Epub 2024 Jan 24. Cell Mol Immunol. 2024. PMID: 38267694 Free PMC article.
References
-
- Kirchhoff F (2010) Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8: 55–67. - PubMed
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418: 646–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

