Scribble modulates the MAPK/Fra1 pathway to disrupt luminal and ductal integrity and suppress tumour formation in the mammary gland
- PMID: 24852022
- PMCID: PMC4031063
- DOI: 10.1371/journal.pgen.1004323
Scribble modulates the MAPK/Fra1 pathway to disrupt luminal and ductal integrity and suppress tumour formation in the mammary gland
Abstract
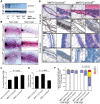
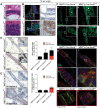
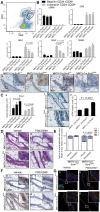
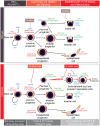
Polarity coordinates cell movement, differentiation, proliferation and apoptosis to build and maintain complex epithelial tissues such as the mammary gland. Loss of polarity and the deregulation of these processes are critical events in malignant progression but precisely how and at which stage polarity loss impacts on mammary development and tumourigenesis is unclear. Scrib is a core polarity regulator and tumour suppressor gene however to date our understanding of Scrib function in the mammary gland has been limited to cell culture and transplantation studies of cell lines. Utilizing a conditional mouse model of Scrib loss we report for the first time that Scrib is essential for mammary duct morphogenesis, mammary progenitor cell fate and maintenance, and we demonstrate a critical and specific role for Scribble in the control of the early steps of breast cancer progression. In particular, Scrib-deficiency significantly induced Fra1 expression and basal progenitor clonogenicity, which resulted in fully penetrant ductal hyperplasia characterized by high cell turnover, MAPK hyperactivity, frank polarity loss with mixing of apical and basolateral membrane constituents and expansion of atypical luminal cells. We also show for the first time a role for Scribble in mammalian spindle orientation with the onset of mammary hyperplasia being associated with aberrant luminal cell spindle orientation and a failure to apoptose during the final stage of duct tubulogenesis. Restoring MAPK/Fra1 to baseline levels prevented Scrib-hyperplasia, whereas persistent Scrib deficiency induced alveolar hyperplasia and increased the incidence, onset and grade of mammary tumours. These findings, based on a definitive genetic mouse model provide fundamental insights into mammary duct maturation and homeostasis and reveal that Scrib loss activates a MAPK/Fra1 pathway that alters mammary progenitor activity to drive premalignancy and accelerate tumour progression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Scribble is required for pregnancy-induced alveologenesis in the adult mammary gland.J Cell Sci. 2016 Jun 15;129(12):2307-15. doi: 10.1242/jcs.185413. Epub 2016 May 13. J Cell Sci. 2016. PMID: 27179074 Free PMC article.
-
Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs.BMC Biol. 2009 Sep 24;7:62. doi: 10.1186/1741-7007-7-62. BMC Biol. 2009. PMID: 19778415 Free PMC article.
-
Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer.Cancer Res. 2014 Jun 1;74(11):3180-94. doi: 10.1158/0008-5472.CAN-13-3415. Epub 2014 Mar 24. Cancer Res. 2014. PMID: 24662921 Free PMC article.
-
Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective.J Mammary Gland Biol Neoplasia. 2010 Mar;15(1):49-63. doi: 10.1007/s10911-010-9168-y. Epub 2010 Jan 27. J Mammary Gland Biol Neoplasia. 2010. PMID: 20101444 Free PMC article. Review.
-
Cell Polarity Proteins in Breast Cancer Progression.J Cell Biochem. 2016 Oct;117(10):2215-23. doi: 10.1002/jcb.25553. Epub 2016 Jun 30. J Cell Biochem. 2016. PMID: 27362918 Review.
Cited by
-
Cooperation of the BTB-Zinc finger protein, Abrupt, with cytoskeletal regulators in Drosophila epithelial tumorigenesis.Biol Open. 2015 Jul 17;4(8):1024-39. doi: 10.1242/bio.012815. Biol Open. 2015. PMID: 26187947 Free PMC article.
-
Modelling Cooperative Tumorigenesis in Drosophila.Biomed Res Int. 2018 Mar 6;2018:4258387. doi: 10.1155/2018/4258387. eCollection 2018. Biomed Res Int. 2018. PMID: 29693007 Free PMC article. Review.
-
The cell polarity protein Scrib functions as a tumor suppressor in liver cancer.Oncotarget. 2017 Apr 18;8(16):26515-26531. doi: 10.18632/oncotarget.15713. Oncotarget. 2017. PMID: 28460446 Free PMC article.
-
Scribble, Erbin, and Lano redundantly regulate epithelial polarity and apical adhesion complex.J Cell Biol. 2019 Jul 1;218(7):2277-2293. doi: 10.1083/jcb.201804201. Epub 2019 May 30. J Cell Biol. 2019. PMID: 31147384 Free PMC article.
-
Cell polarity changes in cancer initiation and progression.J Cell Biol. 2024 Jan 1;223(1):e202308069. doi: 10.1083/jcb.202308069. Epub 2023 Dec 13. J Cell Biol. 2024. PMID: 38091012 Free PMC article. Review.
References
-
- Dow LE, Humbert PO (2007) Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol 262: 253–302. - PubMed
-
- Godde NJ, Galea RC, Elsum IA, Humbert PO (2010) Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia 15: 149–168. - PubMed
-
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, et al. (2008) Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27: 6888–6907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

