Repeat associated non-ATG (RAN) translation: new starts in microsatellite expansion disorders
- PMID: 24852074
- PMCID: PMC4237677
- DOI: 10.1016/j.gde.2014.03.002
Repeat associated non-ATG (RAN) translation: new starts in microsatellite expansion disorders
Abstract
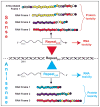
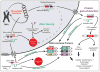
Microsatellite-expansion diseases are a class of neurological and neuromuscular disorders caused by the expansion of short stretches of repetitive DNA (e.g. GGGGCC, CAG, CTG …) within the human genome. Since their discovery 20 years ago, research into how microsatellites expansions cause disease has been examined using the model that these genes are expressed in one direction and that expansion mutations only encode proteins when located in an ATG-initiated open reading frame. The fact that these mutations are often bidirectionally transcribed combined with the recent discovery of repeat associated non-ATG (RAN) translation provides new perspectives on how these expansion mutations are expressed and impact disease. Two expansion transcripts and a set of unexpected RAN proteins must now be considered for both coding and 'non-coding' expansion disorders. RAN proteins have been reported in a growing number of diseases, including spinocerebellar ataxia type 8 (SCA8), myotonic dystrophy type 1 (DM1), Fragile-X tremor ataxia syndrome (FXTAS), and C9ORF72 amyotrophic lateral sclerosis (ALS)/frontotemporal dementia (FTD).
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

