Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age
- PMID: 24852077
- PMCID: PMC11022182
- DOI: 10.1002/14651858.CD004772.pub4
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age
Abstract
Background: In the absence of antiretroviral therapy (ART), over 50% of HIV-infected infants progress to AIDS and death by 2 years of age. However, there are challenges to initiation of ART in early life, including the possibility of drug resistance in the context of prevention of mother-to-child transmission (PMTCT) programs, a paucity of drug choices , uncertain dosing for some medications and long-term toxicities. Key management decisions include when to start ART, what regimen to start, and whether and when to substitute drugs or interrupt therapy. This review, an update of a previous review, aims to summarize the currently available evidence on this topic and inform the ART management in HIV-infected children less than 3 years of age.
Objectives: To evaluate 1) when to start ART in young children (less than 3 years); 2) what ART to start with, comparing first-line non-nucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor (PI)-based regimens; and 3) whether alternative strategies should be used to optimize antiretroviral treatment in this population: induction (initiation with 4 drugs rather than 3 drugs) followed by maintenance ART, interruption of ART and substitution of PI with NNRTI drugs once virological suppression is achieved on a PI-based regimen.
Search methods: Search methodsWe searched for published studies in the Cochrane HIV/AIDS Review Group Trials Register, The Cochrane Library, Pubmed, EMBASE and CENTRAL. We screened abstracts from relevant conference proceedings and searched for unpublished and ongoing trials in clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform).
Selection criteria: We identified RCTs that recruited perinatally HIV-infected children under 3 years of age without restriction of setting. We rejected trials that did not include children less than 3 years of age, did not provide stratified outcomes for those less than 3 years or did not evaluate either timing of ART initiation, choice of drug regimen or treatment switch/interruption strategy.
Data collection and analysis: Two reviewers independently applied study selection criteria, assessed study quality and extracted data. Effects were assessed using the hazard ratio (HR) for time-to-event outcomes, relative risk for dichotomous outcomes and weighted mean difference for continuous outcomes.
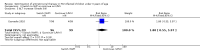
Main results: A search of the databases identified a total of 735 unique, previously unreviewed studies, of which 731 were excluded to leave 4 new studies to incorporate into the review. Four additional studies were identified in conference proceedings, for a total of 8 studies addressing when to start treatment (n=2), what to start (n=3), whether to substitute lopinavir/ritonavir (LPV/r) with nevirapine (NVP) (n=1), whether to use an induction-maintenance ART strategy (n=1) and whether to interrupt treatment (n=1).Treatment initiation in asymptomatic infants with good immunological status was associated with a 75% reduction (HR=0.25; 95%CI 0.12-0.51; p=0.0002) in mortality or disease progression in the one trial with sufficient power to address this question. In a smaller pilot trial, median CD4 cell count was not significantly different between early and deferred treatment groups 12 months after ART.Regardless of previous exposure to nevirapine for PMTCT, the hazard for treatment failure at 24 weeks was 1.79 (95%CI 1.33, 2.41) times higher in children starting ART with a NVP-based regimen compared to those starting with a LPV/r-based regimen (p=0.0001) with no clear difference in the effect observed for children younger or older than 1 year. The hazard for virological failure at 24 weeks was overall 1.84 (95%CI 1.29, 2.63) times higher for children starting ART with a NVP-based regimen compared to those starting with a LPV/r-based regimen (p=0.0008) with a larger difference in time to virological failure (or death) between the NVP and LPV/r-based regimens when ART was initiated in the first year of life.Infants starting a LPV/r regimen and achieving sustained virological suppression who then substituted LPV/r with NVP after median 9 months on LPV/r were less likely to develop virological failure (defined as at least one VL greater than 50 copies/mL) compared with infants who started and stayed on LPV/r (HR=0.62, 95%CI 0.41, 0.92, p=0.02). However the hazard for confirmed failure at a higher viral load (>1000 copies/mL) was greater among children who switched to NVP compared to those who remained on LPV/r (HR=10.19, 95% CI 2.36, 43.94, p=0.002).Children undergoing an induction-maintenance ART approach with a 4-drug NNRTI-based regimen for 36 weeks, followed by 3-drug ART, had significantly greater CD4 rise than children receiving a standard 3-drug NNRTI-based ART at 36 weeks (mean difference 1.70 [95%CI 0.61, 2.79] p=0.002) and significantly better viral load response at 24 weeks (OR 1.99 [95%CI 1.09, 3.62] p=0.02). However, the immunological and virological benefits were short-term.The one trial of treatment interruption that compared children initiating continuous ART from infancy with children interrupting ART was terminated early because the duration of treatment interruption was less than 3 months in most infants. Children interrupting treatment had similar growth and occurrence of serious adverse events as those in the continuous arm.
Authors' conclusions: ART initiation in asymptomatic children under 1 year of age reduces morbidity and mortality, but it remains unclear whether there are clinical benefits to starting ART in asymptomatic children diagnosed with HIV infection between 1-3 years.The available evidence shows that a LPV/r-based first-line regimen is more efficacious than a NVP-based regimen, regardless of PMTCT exposure status. New formulations of LPV/r are urgently required to enable new WHO recommendations to be implemented. An alternative approach to long-term LPV/r is substituting LPV/r with NVP once virological suppression is achieved. This strategy looked promising in the one trial undertaken, but may be difficult to implement in the absence of routine viral load testing.A 4-drug induction-maintenance approach showed short-term virological and immunological benefits during the induction phase but, in the absence of sustained benefits, is not recommended as a routine treatment strategy. Treatment interruption following early ART initiation in infancy was challenging for children who were severely immunocompromised in the context of poor clinical immunological condition at ART initiation due to the short duration of interruption, and is therefore not practical in ART treatment programmes where close monitoring is not feasible.
Conflict of interest statement
AJP is a co‐investigator on the PEHSS and ARROW trials. EJA is a co‐investigator on the P1060 and NEVEREST trials.
Figures
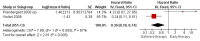
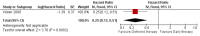




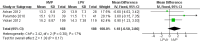
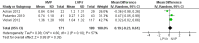








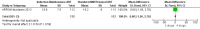
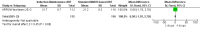

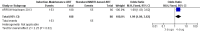


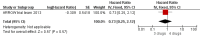
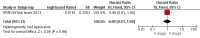



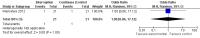





























Update of
-
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004772. doi: 10.1002/14651858.CD004772.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. PMID: 22786492 Updated.
Similar articles
-
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004772. doi: 10.1002/14651858.CD004772.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. PMID: 22786492 Updated.
-
Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women.Cochrane Database Syst Rev. 2010 Mar 17;2010(3):CD008440. doi: 10.1002/14651858.CD008440. Cochrane Database Syst Rev. 2010. PMID: 20238370 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2016 Dec 10;12(12):CD004246. doi: 10.1002/14651858.CD004246.pub4. Cochrane Database Syst Rev. 2016. PMID: 27943261 Free PMC article.
Cited by
-
Early antiretroviral therapy is protective against epilepsy in children with human immunodeficiency virus infection in botswana.J Acquir Immune Defic Syndr. 2015 Jun 1;69(2):193-9. doi: 10.1097/QAI.0000000000000563. J Acquir Immune Defic Syndr. 2015. PMID: 25647527 Free PMC article.
-
Mega-map of systematic reviews and evidence and gap maps on the interventions to improve child well-being in low- and middle-income countries.Campbell Syst Rev. 2020 Oct 28;16(4):e1116. doi: 10.1002/cl2.1116. eCollection 2020 Dec. Campbell Syst Rev. 2020. PMID: 37018457 Free PMC article.
-
'First 1000 days' health interventions in low- and middle-income countries: alignment of South African policies with high-quality evidence.Glob Health Action. 2017;10(1):1340396. doi: 10.1080/16549716.2017.1340396. Glob Health Action. 2017. PMID: 28715934 Free PMC article.
-
Using CD4 percentage and age to optimize pediatric antiretroviral therapy initiation.Pediatrics. 2014 Oct;134(4):e1104-16. doi: 10.1542/peds.2014-0527. Pediatrics. 2014. PMID: 25266426 Free PMC article. Clinical Trial.
-
CD4+ cell count recovery following initiation of HIV antiretroviral therapy in older childhood and adolescence.AIDS. 2018 Sep 10;32(14):1977-1982. doi: 10.1097/QAD.0000000000001905. AIDS. 2018. PMID: 29927784 Free PMC article.
References
References to studies included in this review
Achan 2012 {published and unpublished data}
-
- Achan j, Kahuru A, Ikilezi G, Ruel T, Clark T, Charlebois E, Rosenthal P, Dorsey G, Havlir D, Kamya M. Significant Reduction in Risk of Malaria among HIV+ Children Receiving Lopinavir/ritonavir‐based ART Compared to NNRTI‐based ART, a Randomized Open‐label Trial. March 2012; Vol. CROI.
ARROW trial team 2013 {published and unpublished data}
-
- ARROW Trial team. Routine versus clinically driven laboratory monitoring and first‐line antiretroviral therapy strategies in Africanchildren with HIV (ARROW): a 5‐year open‐label randomised factorial trial [Routine versus clinically driven laboratory monitoring and first‐line antiretroviral therapy strategies in Africanchildren with HIV (ARROW): a 5‐year open‐label randomised factorial trial]. Lancet. 2013 Mar 6;370(12):62198‐9. - PMC - PubMed
Coovadia 2010 {published data only}
Palumbo 2010 {published and unpublished data}
-
- Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, Bwakura‐Dangarembizi M, Chi BH, Musoke P, Kamthunzi P, Schimana W, Purdue L, Eshleman SH, Abrams EJ, Millar L, Petzold E, Mofenson LM, Jean‐Philippe P, Violari A. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010;363(16):1510‐20. - PMC - PubMed
Prendergast 2008 (a) {published and unpublished data}
-
- Prendergast A, Mphatswe W, Tudor‐Williams G, Rakgotho M, Pillay V, Thobakgale C, McCarthy N, Morris L, Walker BD, Goulder P. Early virological suppression with three‐class antiretroviral therapy in HIV‐infected AFrican infants. AIDS 2008;22(11):1333‐43. - PubMed
Violari 2008 {published data only}
Violari 2012 {published and unpublished data}
-
- Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow‐Mosha L, Kamthunzi P, Chi BH, Cotton MF, Moultrie H, Khadse S, Schimana W, Bobat R, Purdue L, Eshleman SH, Abrams EJ, Millar L, Petzold E, Mofenson LM, Jean‐Philippe P, Palumbo P. NVP‐ vs LPV/r‐based ART among HIV+ Infants in Resource‐limited Settings: The IMPAACT P1060 Trial [Nevirapine versus ritonavir‐boosted lopinavir for HIV‐infected children.]. N Engl J Med. 2012 Jun 21; Vol. 366, issue 25:2380‐9. - PMC - PubMed
Wamalwa 2012 {published data only (unpublished sought but not used)}
-
- Wamalwa D, Benki‐Nugent S, Langat A, Tapia K, Ngugi E, Moraa H, Otieno V, Richardson B, Overbaugh J, John‐Stewart G. [Treatment Interruption in Infants following 24 Months of Empiric ART: Kenya]. 19th Conference on Retroviruses and Opportunistic Infections‐ Seattle, Washington State (US). March, 2012.
References to studies excluded from this review
Ananworanich 2008 {published data only}
-
- Ananworanich J, Kosalaraksa P, Siangphoe U, Engchanil C, Pancharoen C, Lumbiganon P, Intasan J, Apateerapong W, Chuenyam T, Ubolyam S, Bunupuradah T, Lange J, Cooper DA, Phanuphak P, HIV‐NAT 010 Study Team. A feasibility study of immediate versus deferred antiretroviral therapy in children with HIV infection. AIDS Res Ther 2008;5:24. - PMC - PubMed
Cotton 2012 {published data only}
-
- Cotton M, Violari A, Gibb G, Otwombe K, Josipovic D, Panchia R, Jean‐Philippe P, Handelsman E, McIntyre J, Babiker A and the CHER Team. Early ART followed by Interruption Is Safe and Is Associated with Better Outcomes than Deferred ART in HIV+ Infants: Final Results from the 6‐Year Randomized CHER Trial, South Africa. CROI 2012. March 2012; Vol. 28 LB.
King 2005 {published data only}
-
- King JR, Nachman S, Yogev R, Hodge J, Aldrovandi G, Hughes MD, Chen J, Wiznia A, Damle B, Acosta EP. Efficacy, tolerability and pharmacokinetics of two nelfinavir‐based regimens in human immunodeficiency virus‐infected children and adolescents: pediatric AIDS clinical trials group protocol 403. Pediatr Infect Dis J 2005;24(10):880‐5. - PubMed
Krogstad 2002 {published data only}
-
- Krogstad P, Lee S, Johnson G, Stanley K, McNamara J, Moye J, Jackson JB, Aguayo R, Dieudonne A, Khoury M, Mendez H, Nachman S, Wiznia A, Pediatric AIDS Clinical Trials Group 377 Study Team. Nucleoside‐analogue reverse‐transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis 2002;34(7):991‐1001. - PubMed
Lockman 2007 {published data only}
-
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med 2007;356(2):135‐47. - PubMed
Luzuriaga 2004 {published data only}
-
- Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL, PACTG 356 Investigators. A trial of three antiretroviral regimens in HIV‐1‐infected children. N Engl J Med 2004;350(24):2471‐80. - PubMed
NCT00427297 {unpublished data only}
-
- John‐Stewart G. Optimizing Pediatric HIV‐1 Treatment in Infants With Prophylactic Exposure to Nevirapine, Nairobi, Kenya. Clinicaltrials.gov NCT00427297.
PENPACT1 study team {published data only (unpublished sought but not used)}
-
- The PENPACT‐1 (PENTA 9/PACTG 390) Study Team. First‐line antiretroviral therapy with a protease inhibitor versus non‐nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV‐infected children: an open‐label, randomised phase 2/3 trial. The Lancet Infectious Diseases 2011;11(4):273‐283. - PMC - PubMed
Prendergast 2008 (b) {published data only}
-
- Prendergast A, Chonco F, Tudor‐Williams G, Mphatswe W, Cengimbo A, Thobakgale C, Dong C, Coovadia H, Walker B, Goulder P. Randomized controlled trial of 3 approaches to management of HIV‐infected infants [Randomized controlled trial of 3 approaches to management of HIV‐infected infants]. 16th Conferences on Retroviruses and Opportunistic Infections, Boston, Massachusetts, USA Canada 3‐6 February 2008;Abstract 77LB.
Puthanakit 2012 {published data only}
-
- Puthanakit T, Saphonn V, Ananworanich J, Kosalaraksa P, Hansudewechakul R, Vibol U, Kerr SJ, Kanjanavanit S, Ngampiyaskul C, Wongsawat J, Luesomboon W, Ngo‐Giang‐Huong N, Chettra K, Cheunyam T, Suwarnlerk T, Ubolyam S, Shearer WT, Paul R, Mofenson LM, Fox L, Law MG, Cooper DA, Phanuphak P, Chhi Vun M, Ruxrungtham K, on behalf of the PREDICT Study Group. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre,randomised, open‐label trial. The Lancet ID October 9, 2012;S1473‐3099(12):70242‐6. - PMC - PubMed
Wiznia 2000 {published data only}
-
- Wiznia A, Stanley K, Krogstad P, Johnson G, Lee S, McNamara J, Moye J, Jackson JB, Mendez H, Aguayo R, Dieudonne A, Kovacs A, Bamji M, Abrams E, Rana S, Sever J, Nachman S. Combination nucleoside analogue reverse transcriptase inhibitor(s) plus nevirapine, nelfinavir, or ritonavir in stable antiretroviral therapy‐experienced HIV‐infected children: week 24 results of a randomized controlled trial‐‐PACTG 377. Pediatric AIDS Clinical Trials Group 377 Study Team. AIDS Res Hum Retroviruses 2000;16(12):1113‐21. - PubMed
Wongsawat 2010 {published data only}
-
- Wongsawat J, Puthanakit T, Kanjanavanit S, Hansudewechakul R, Ngampiyaskul C, Kerr SJ, Ubolyam S, Suwanlerk T, Kosalaraksa P, Luesomboon W, Ngo‐Giang‐Huong N, Chandara M, Saphonn V, Ruxrungtham K, Ananworanich J, PREDICT Study Group. CD4 cell count criteria to determine when to initiate antiretroviral therapy in human immunodeficiency virus‐infected children. Pediatr Infect Dis J 2010;29(10):966‐8. - PMC - PubMed
References to ongoing studies
ANRS 12206‐ MONOD {published data only}
-
- Leroy V, Timite‐Konan M, Mutabazi V, Meda N. International phase 2b‐3 randomized clinical trial to assess two once‐daily simplified antiretroviraltriple therapies among HIV‐infected children early treated by a 12‐month twice daily triple therapybetween 6 weeks and 24 months of age and in virological success in Africa:the MONOD Project(Burkina Faso, Ivory Coast, Rwanda). clinicaltrial.gov NCT01127204.
NCT01146873‐NEVEREST 3 {unpublished data only}
-
- Treatment Options for Protease Inhibitor‐exposed Children (NEVEREST‐III). Ongoing study June 2010 (expected completion September 2014).
Additional references
3Cs4kids 2008
-
- Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee. Epidemiology and Social Markers for predicting mortality in untreated HIV‐infected children in resource‐limited settings: a meta‐analysis. AIDS 2 January 2008;22(1):97‐105. [http://journals.lww.com/aidsonline/Fulltext/2008/01020/Markers_for_predi... - PubMed
Apollo 2013
-
- Apollo T, Zinyowera S, Dzangare J, Chakanyuka C, Mugurungi O, Penazzato M, Martin E, Jordan M, Perriens J, Bertagnolio S. [World Health Organization HIV Drug Resistance surveillance in children less than 18 months newly diagnosed with HIV in Zimbabwe]. 7th IAS Conference in HIV pathogenesis. treatment and prevention. Luala Lumpur, Malaysia. 30 June.
Arpadi 2013
Arrive 2007
-
- Arrivé E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, Leroy V, Perre PV, Rouzioux C, Dabis F, Ghent Group on HIV in Women and Children. Prevalence of resistance to nevirapine in mothers and children after single‐dose exposure to prevent vertical transmission of HIV‐1: a meta‐analysis.. Int J Epidemiol 2007 Oct;36(5):1009‐21. [http://www.ncbi.nlm.nih.gov/pubmed/17533166?itool=EntrezSystem2.PEntrez.... - PubMed
Chatterjee 2010
Church 2009
-
- Church JD, Mwatha A, Bagenda D, Omer SB, Donnell D, Musoke P, Nakabiito C, Eure C, Bakaki P, Matovu F, Thigpen MC, Guay LA, McConnell M, Fowler MG, Jackson JB, Eshleman SH. In utero HIV infection is associated with an increased risk of nevirapine resistance in ugandan infants who were exposed to perinatal single dose nevirapine.. AIDS Res Hum Retroviruses 2009 Jul;25(7):673‐7.. [http://www.ncbi.nlm.nih.gov/pubmed/19552593?itool=EntrezSystem2.PEntrez.... - PMC - PubMed
DHHS 2011
-
- Department of Health and Human Services. Antiretroviral Therapy and Medical Management of HIV‐ Infected Children. 2011.
Dunn 2008
-
- Dunn D, Woodburn P, Duong T, Peto J, Phillips A, Gibb D, Porter K, HIV Paediatric Prognostic Markers Collaborative Study (HPPMCS), Concerted Action on Sero‐Conversion to AIDS and Death in Europe (CASCADE) Collaboration. Current CD4 cell count and the short‐term risk of AIDS and death before the availability of effective antiretroviral therapy in HIV‐infected children and adults. [Current CD4 cell count and the short‐term risk of AIDS and death before the availability of effective antiretroviral therapy in HIV‐infected children and adults.]. J Infect Dis 2008 Feb 1;197(3):398‐404. [http://www.ncbi.nlm.nih.gov/pubmed/18248303?ordinalpos=19&itool=Entr... - PubMed
EPPICC 2011
Ferrand 2009
Fillekes 2013
-
- Fillekes Q, Mulenga V, Kabamba D, Kankasa C, Thomason MJ, Cook A, Chintu C, Gibb DM, Walker AS, Burger DM, on behalf of the CHAPAS‐1 trial team. [Is nevirapine dose escalation appropriate in young African HIV+ children?]. AIDS. 2013 Apr 16 [Epub ahead of print]. - PubMed
Gortmaker 2001
-
- Gortmaker SL, Hughes M, Cervia J, Brady M, Johnson GM, Seage GR 3rd, Song LY, Dankner WM, Oleske JM, Pediatric AIDS Clinical Trials Group Protocol 219 Team. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV‐1. N Engl J Med. 2001 Nov 22;345(21):1522‐8. - PubMed
Griffiths 1976
-
- Griffiths R. The Abilities of Babies. University of London Press; A study in mental measurement 1976:pp. 5–44.
Gupta 2012
-
- Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, Sawyer AW, Hamers RL, Ndembi N, Pillay D, Bertagnolio S. Global trends in antiretroviral resistance in treatment‐naive individuals with HIV after rollout of antiretroviral treatment in resource‐limited settings: a global collaborative study and meta‐regression analysis. Lancet 2012 Oct 6;380(9849):1250‐8. - PMC - PubMed
Higgins 2011
-
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. March 2011.
HPPMCS 2003
-
- Dunn D, HIV Paediatric Prognostic Markers Collaborative Study Group. Short‐term risk of disease progression in HIV‐1‐infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta‐analysis. [Short‐term risk of disease progression in HIV‐1‐infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta‐analysis.]. Lancet Nov 15;362(9396):1605‐11.. [http://www.ncbi.nlm.nih.gov/pubmed/14630440] - PubMed
Hunt 2011
-
- Hunt GM, Coovadia A, Abrams EJ, Sherman G, Meyers T, Morris L, Kuhn L. HIV‐1 drug resistance at antiretroviral treatment initiation in children previously exposed to single‐dose nevirapine. AIDS 2011 Jul 31;25(12):1461‐1469. [http://www.ncbi.nlm.nih.gov/pubmed/21633285] - PMC - PubMed
Italian Register 1999
-
- The Italian Register for HIV Infection in Children. Rapid disease progression in HIV‐1 perinatally infected children born to mothers receiving zidovudine monotherapy during pregnancy.. AIDS 1999;13(8):927‐33. - PubMed
Jaquet 2000
-
- Jaquet D, Lévine M, Ortega‐Rodriguez E, Faye A, Polak M, Vilmer E, Lévy‐Marchal C. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV‐infected children. AIDS 2000 Sep 29;14(14):2123‐8.. [http://www.ncbi.nlm.nih.gov/pubmed?term=jaquet%202000%20HIV] - PubMed
Karchava 2006
-
- Karchava M, Pulver W, Smith L, Philpott S, Sullivan TJ, Wethers J, Parker MM.SourceWadsworth Center, New York State Department of Health, Albany, NY 12201‐2002, USA. Prevalence of drug‐resistance mutations and non‐subtype B strains among HIV‐infected infants from New York State. J Acquir Immune Defic Syndr 2006 Aug 15;42(5):614‐9. [http://www.ncbi.nlm.nih.gov/pubmed/16868498?ordinalpos=3&itool=Entre... - PMC - PubMed
Kinabo 2013
-
- Kinabo GD, Sprengers M, Msuya LJ, et al. Prevalence of Lipodystrophy in HIV‐Infected Children in Tanzania on HAART. The Pediatric Infectious Disease Journal 2013;32:39‐44. - PubMed
Kuhn 2012
Kuhn 2013
-
- Kuhn L, Hunt G, Technau K, Coovadia A, Black V, Morris L, Abrams E. and Finding Infants with HIV Disease and Evaluating Resistance Study Group. [Pre‐treatment Drug Resistance Mutations among HIV+ Children <2 Years of Age Who Failed or Missed PMTCT: Johannesburg, South Africa]. 20th Conference on Retroviruses and Opportunistic Infections‐ Atlanta, Georgia. 3‐6 March 2013; Vol. Abstract 951.
Laughton 2012
Lewis 2012
-
- Lewis J, Walker AS, Castro H, Rossi A, Gibb DM, Giaquinto C, Klein N, Callard R. Age and CD4 count at initiation of antiretroviral therapy in HIV‐infected children: effects on long‐term T‐cell reconstitution.. J Infect Dis 2012 Feb 15;205(4):548‐56.. - PubMed
Lindsey 2012
-
- Lindsey J, Hughes M, A Violari, Eshleman S, Abrams E, Mofenson L, Jean‐Philippe P, Palumbo P and P1060 Study Team . Predictors of Virologic and Clinical Response to Nevirapine‐ vs Lopinavir/ritonavir‐based ART in Infants and Children with and without Prior Nevirapine Exposure for the PMTCT. CROI 2012. March 2012; Vol. paper 25.
Lockman 2007
-
- Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. Response to Antiretroviral Therapy after a Single, Peripartum Dose of Nevirapine. N Engl J Med January 11, 2007, (356):135‐147. [http://www.nejm.org/doi/full/10.1056/NEJMoa062876] - PubMed
Luiz 2001
-
- Luiz D, Foxcroft C, Stewart R. The construct validity of the Griffiths Scales of Infant Development.. Child: care health and development. 2001;27:73–83. - PubMed
Martinson 2007
-
- Martinson NA, Morris L, Gray G, Moodley D, Pillay V, Cohen S, Dhlamini P, Puren A, Bhayroo S, Steyn J, McIntyre JA. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):148‐53.Selection and persistence of viral resistance in HIV‐infected children after exposure to single‐dose nevirapine.. J Acquir Immune Defic Syndr 2007 Feb 1;44(2):148‐53. [http://www.ncbi.nlm.nih.gov/pubmed/17117145?itool=EntrezSystem2.PEntrez.... - PubMed
McComsey 2002
-
- McComsey G. Update on mitochondrial toxicity of antiretrovirals and its link to lipodystrophy.. AIDS Rev 2002 Jul‐Sep;4(3):140‐7. [http://www.ncbi.nlm.nih.gov/pubmed/12416448] - PubMed
McIntosh 1996
-
- McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, Burchett SK. .Age‐ and time‐related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J 1996 Dec;15(12):1087‐91. [http://www.ncbi.nlm.nih.gov/pubmed/8970217?itool=EntrezSystem2.PEntrez.P... - PubMed
Mphatswe 2007
-
- Mphatswe W, Blanckenberg N, Tudor‐Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, McCarthy N, Walker BD, Kiepiela P, Goulder P. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis.. AIDS 2007;21(10):1253‐61. - PubMed
Mulenga 2010
-
- Mulenga V, Cook A, Walker AS, Kabamba D, Chijoka C, Ferrier A, Kalengo C, Kityo C, Kankasa C, Burger D, Thomason M, Chintu C, Gibb DM. Strategies for nevirapine initiation in HIV‐infected children taking pediatric fixed‐dose combination "baby pills" in Zambia: a randomized controlled trial.. Clin Infect Dis. 2010 Nov 1;51(9):1081‐9. - PubMed
Musiime 2009
-
- Musiime V, Ssali F, Kayiwa J, Namala W, Kizito H, Kityo C, Mugyenyi P. Response to nonnucleoside reverse transcriptase inhibitor‐based therapy in HIV‐infected children with perinatal exposure to single‐dose nevirapine. AIDS Res Hum Retroviruses 2009 Oct;25(10):989‐96. [http://www.ncbi.nlm.nih.gov/pubmed/19778270] - PubMed
Newell 2004
-
- Newell ML, Coovadia H, Cortina‐Borja M, Rollins N, Gaillard P, Dabis F, Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV‐infected mothers in Africa: a pooled analysis.. Lancet 2004 Oct 2‐8;364(9441):1236‐43. - PubMed
NIAID 2009
-
- United States National Institute of Allergy and Infectious Diseases (NIAID). Division of AIDS, Table for Grading Adult and Pediatric Adverse Events (Toxicity Table),dated December 2004 (Clarification August 2009) [Available from: hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Table_for_Grading_....
PENTA 11
-
- Paediatric European Network for Treatment of AIDS. Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 2010 Jan 16;24(2):231‐41. - PubMed
PENTA 2002
-
- Paediatric European Network for Treatment of AIDS (PENTA). Comparison of dual nucleoside‐analogue reverse‐transcriptase inhibitor regimens with and without nelfinavir in children with HIV‐1 who have not previously been treated: the PENTA 5 randomised trial [Comparison of dual nucleoside‐analogue reverse‐transcriptase inhibitor regimens with and without nelfinavir in children with HIV‐1 who have not previously been treated: the PENTA 5 randomised trial]. Lancet 2002 Mar;2(359(9308)):733‐40. - PubMed
PENTA 2009
-
- PENTA Steering Committee. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV‐1 infection.. HIV Medicine 2009;10(10):591‐613. - PubMed
Persaud 2007
-
- Persaud D, Palumbo P, Ziemniak C, Chen J, Ray SC, Hughes M, Havens P, Purswani M, Gaur AH, Chadwick EG, Pediatric AIDS Clinical Trials Group P1030 Team. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor‐resistant HIV‐1 among recently infected infants born in the United States.. J Infect Dis 2007 May 15;195(10):1402‐10.. [http://www.ncbi.nlm.nih.gov/pubmed/17436219?ordinalpos=2&itool=Entre... - PubMed
Persaud 2013
Pillay 2008
-
- Pillay V, Ledwaba J, Hunt G, Rakgotho M, Singh B, Makubalo L, Bennett DE, Puren A, Morris L. Antiretroviral drug resistance surveillance among drug‐naive HIV‐1‐infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther. ; 2008;13(Suppl 2):101‐7. [http://www.ncbi.nlm.nih.gov/pubmed/18575198] - PubMed
Piloya 2012
Prendergast 2012
Ruel 2013
-
- Ruel T, Kakuru A, Ikilezi G, Mwangwa F, Dorsey G, Rosenthal P, Charlebois E, Havlir D, Kamya M, Achan J. [Comparison of Virologic and Immunologic Outcomes between HIV+ Ugandan Children Randomized to Ritonavir‐boosted Lopinavir or NNRTI‐ based ART]. 20th Conference on Retroviruses and Opportunistic Infections‐ Atlanta, Georgia, USA.. 3‐6 March 2013; Vol. Abstract 88 LB.
UNAIDS 2012
-
- UNAIDS. UNAIDS Report on the global AIDS epidemIc 2012. http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/g... 2012.
Vigano 2003
-
- Viganò A, Mora S, Testolin C, Beccio S, Schneider L, Bricalli D, Vanzulli A, Manzoni P, Brambilla P. Increased lipodystrophy is associated with increased exposure to highly active antiretroviral therapy in HIV‐infected children. J Acquir Immune Defic Syndr 2003 Apr 15;32(5):482‐9. [http://www.ncbi.nlm.nih.gov/pubmed/12679698] - PubMed
Wade 1994
-
- Wade AM, Ades AE. Age‐related reference ranges: significance tests for models and confidence intervals for centiles. Stat Med 1994 Nov 30;13(22):2359‐67.. [http://www.ncbi.nlm.nih.gov/pubmed/7855469?itool=EntrezSystem2.PEntrez.P... - PubMed
Walker 2004
-
- Walker AS, Doerholt K, Sharland M, Gibb DM, Collaborative HIV Paediatric Study (CHIPS) Steering Committee. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS 2004 Sep 24;18(14):1915‐24. [http://www.ncbi.nlm.nih.gov/pubmed/15353977?ordinalpos=27&itool=Entr... - PubMed
WHO 2010
-
- WHO. Antiretroviral therapy for HIV infection in infants and children: Recommendations for a public health approach (2010 revision). 2010; Vol. http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf. - PubMed
WHO 2011
-
- WHO 2011. GLOBAL HIV/AIDS RESPONSE. Epidemic update and health sector progress towards Universal Access. Progress Report 2011. [http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf]
WHO 2013
-
- World Health Organisation. Consolidated Guidelines on the use of Antiretroviral drugs for treating and preventing HIVinfection. June 2013.
References to other published versions of this review
Penazzato 2012
-
- Penazzato M, Prendergast A, Tierney J, Cotton M, Gibb D. Effectiveness of antiretroviral therapy in HIV‐infected children under 2 years of age. Cochrane Database of Systematic Reviews 2012 Jul 11, Issue 7. [PUBMED: 22786492] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

