Improving the distribution of Doxil® in the tumor matrix by depletion of tumor hyaluronan
- PMID: 24852095
- PMCID: PMC4156903
- DOI: 10.1016/j.jconrel.2014.05.019
Improving the distribution of Doxil® in the tumor matrix by depletion of tumor hyaluronan
Abstract
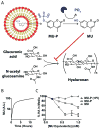
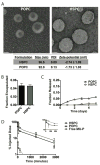
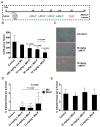
Liposomes improve the pharmacokinetics and safety of rapidly cleared drugs, but have not yet improved the clinical efficacy compared to the non-encapsulated drug. This inability to improve efficacy may be partially due to the non-uniform distribution of liposomes in solid tumors. The tumor extra-cellular matrix is a barrier to distribution and includes the high molecular weight glycosaminoglycan, hyaluronan (HA). Strategies to remove HA or block its synthesis may improve drug delivery into solid tumors. Orally administered methylumbelliferone (MU) is an inhibitor of HA synthesis, but it is limited by low potency and limited solubility. In this study, we encapsulate a water-soluble phosphorylated prodrug of MU (MU-P) in a liposome (L-MU-P). We demonstrate that L-MU-P is a more potent inhibitor of HA synthesis than oral MU in the 4T1 murine mammary carcinoma model using both a quantitative ELISA and histochemistry. We show that HA depletion improves the tumor distribution of liposomes computed using Mander's colocalization analysis of liposomes with the tumor vasculature. Hyaluronan depletion also increases the fraction of the tumor area positive for liposomes. This improved distribution extends the overall survival of mice treated with Doxil®.
Keywords: Breast cancer; Extra-cellular matrix; Liposomes; Tumor penetration.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
F.C.S. declares a conflict of interest due to his involvement in a liposome company. The other authors declare no conflict of interest.
Figures




Similar articles
-
Hyaluronated liposomes containing H2S-releasing doxorubicin are effective against P-glycoprotein-positive/doxorubicin-resistant osteosarcoma cells and xenografts.Cancer Lett. 2019 Aug 1;456:29-39. doi: 10.1016/j.canlet.2019.04.029. Epub 2019 Apr 29. Cancer Lett. 2019. PMID: 31047947
-
Doxorubicin-loaded nanocarriers: A comparative study of liposome and nanostructured lipid carrier as alternatives for cancer therapy.Biomed Pharmacother. 2016 Dec;84:252-257. doi: 10.1016/j.biopha.2016.09.032. Epub 2016 Sep 21. Biomed Pharmacother. 2016. PMID: 27664949
-
Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin.J Control Release. 2015 Dec 28;220(Pt A):275-286. doi: 10.1016/j.jconrel.2015.10.044. Epub 2015 Oct 27. J Control Release. 2015. PMID: 26518722
-
Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside.Adv Drug Deliv Rev. 2020;154-155:13-26. doi: 10.1016/j.addr.2020.07.027. Epub 2020 Aug 7. Adv Drug Deliv Rev. 2020. PMID: 32777239 Review.
-
In vitro and in vivo characterizations of PEGylated liposomal doxorubicin.Bioanalysis. 2011 Feb;3(3):333-44. doi: 10.4155/bio.10.204. Bioanalysis. 2011. PMID: 21320053 Review.
Cited by
-
Peptide mediated active targeting and intelligent particle size reduction-mediated enhanced penetrating of fabricated nanoparticles for triple-negative breast cancer treatment.Oncotarget. 2015 Dec 1;6(38):41258-74. doi: 10.18632/oncotarget.5692. Oncotarget. 2015. PMID: 26517810 Free PMC article.
-
Remodeling Components of the Tumor Microenvironment to Enhance Cancer Therapy.Front Oncol. 2015 Oct 14;5:214. doi: 10.3389/fonc.2015.00214. eCollection 2015. Front Oncol. 2015. PMID: 26528429 Free PMC article. Review.
-
Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors.ACS Nano. 2019 Oct 22;13(10):11008-11021. doi: 10.1021/acsnano.9b02395. Epub 2019 Sep 20. ACS Nano. 2019. PMID: 31503443 Free PMC article.
-
Photodynamic Therapy: A Novel Approach for Head and Neck Cancer Treatment with Focusing on Oral Cavity.Biol Proced Online. 2024 Aug 17;26(1):25. doi: 10.1186/s12575-024-00252-3. Biol Proced Online. 2024. PMID: 39154015 Free PMC article. Review.
-
Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway.ACS Nano. 2018 Nov 27;12(11):11041-11061. doi: 10.1021/acsnano.8b05189. Epub 2018 Oct 16. ACS Nano. 2018. Retraction in: ACS Nano. 2021 Jun 22;15(6):10735. doi: 10.1021/acsnano.0c08653. PMID: 30481959 Free PMC article. Retracted.
References
-
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. - PubMed
-
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. - PubMed
-
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

