Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males
- PMID: 24852170
- PMCID: PMC4031044
- DOI: 10.1371/journal.pgen.1004356
Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males
Abstract
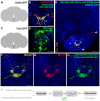
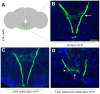
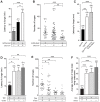
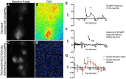
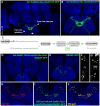
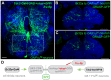
Chemosensory pheromonal information regulates aggression and reproduction in many species, but how pheromonal signals are transduced to reliably produce behavior is not well understood. Here we demonstrate that the pheromonal signals detected by Gr32a-expressing chemosensory neurons to enhance male aggression are filtered through octopamine (OA, invertebrate equivalent of norepinephrine) neurons. Using behavioral assays, we find males lacking both octopamine and Gr32a gustatory receptors exhibit parallel delays in the onset of aggression and reductions in aggression. Physiological and anatomical experiments identify Gr32a to octopamine neuron synaptic and functional connections in the suboesophageal ganglion. Refining the Gr32a-expressing population indicates that mouth Gr32a neurons promote male aggression and form synaptic contacts with OA neurons. By restricting the monoamine neuron target population, we show that three previously identified OA-Fru(M) neurons involved in behavioral choice are among the Gr32a-OA connections. Our findings demonstrate that octopaminergic neuromodulatory neurons function as early as a second-order step in this chemosensory-driven male social behavior pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Suppression of male courtship by a Drosophila pheromone receptor.Nat Neurosci. 2008 Aug;11(8):874-6. doi: 10.1038/nn.2161. Epub 2008 Jul 20. Nat Neurosci. 2008. PMID: 18641642 Free PMC article.
-
A subset of octopaminergic neurons are important for Drosophila aggression.Nat Neurosci. 2008 Sep;11(9):1059-67. doi: 10.1038/nn.2164. Nat Neurosci. 2008. PMID: 19160504
-
A Circuit Node that Integrates Convergent Input from Neuromodulatory and Social Behavior-Promoting Neurons to Control Aggression in Drosophila.Neuron. 2017 Aug 30;95(5):1112-1128.e7. doi: 10.1016/j.neuron.2017.08.017. Neuron. 2017. PMID: 28858617 Free PMC article.
-
Aggression and courtship in Drosophila: pheromonal communication and sex recognition.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Nov;199(11):1065-76. doi: 10.1007/s00359-013-0851-5. Epub 2013 Sep 17. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 24043358 Free PMC article. Review.
-
Neurogenetics of courtship and mating in Drosophila.Adv Genet. 2008;62:67-184. doi: 10.1016/S0065-2660(08)00603-2. Adv Genet. 2008. PMID: 19010254 Review.
Cited by
-
Sensitivity to expression levels underlies differential dominance of a putative null allele of the Drosophila tβh gene in behavioral phenotypes.PLoS Biol. 2021 May 10;19(5):e3001228. doi: 10.1371/journal.pbio.3001228. eCollection 2021 May. PLoS Biol. 2021. PMID: 33970909 Free PMC article.
-
Molecular mechanisms and the conflict between courtship and aggression in three-spined sticklebacks.Mol Ecol. 2016 Sep;25(17):4368-76. doi: 10.1111/mec.13766. Epub 2016 Aug 26. Mol Ecol. 2016. PMID: 27452346 Free PMC article.
-
Characterization of the Sexually Dimorphic fruitless Neurons That Regulate Copulation Duration.Front Physiol. 2018 Jun 25;9:780. doi: 10.3389/fphys.2018.00780. eCollection 2018. Front Physiol. 2018. PMID: 29988589 Free PMC article.
-
Serotonin and the search for the anatomical substrate of aggression.Fly (Austin). 2014;8(4):200-5. doi: 10.1080/19336934.2015.1045171. Epub 2015 Apr 29. Fly (Austin). 2014. PMID: 25923771 Free PMC article.
-
Polymorphism and division of labour in a socially complex ant: neuromodulation of aggression in the Australian weaver ant, Oecophylla smaragdina.Proc Biol Sci. 2015 Jul 22;282(1811):20150704. doi: 10.1098/rspb.2015.0704. Proc Biol Sci. 2015. PMID: 26136448 Free PMC article.
References
-
- Dahanukar A, Ray A (2010) Courtship, aggression and avoidance: Pheromones, receptors and neurons for social behaviors in Drosophila. Fly (Austin) 5: 58–63. - PubMed
-
- Ferrero DM, Liberles SD (2010) The secret codes of mammalian scents. Wiley Interdiscip Rev Syst Biol Med 2: 23–33. - PubMed
-
- Birmingham JT, Tauck DL (2003) Neuromodulation in invertebrate sensory systems: from biophysics to behavior. J Exp Biol 206: 3541–3546. - PubMed
-
- Farooqui T (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32: 1511–1529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

