STRA6 is critical for cellular vitamin A uptake and homeostasis
- PMID: 24852372
- PMCID: PMC4168826
- DOI: 10.1093/hmg/ddu258
STRA6 is critical for cellular vitamin A uptake and homeostasis
Abstract
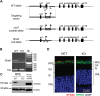
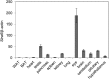
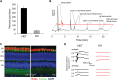
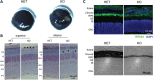
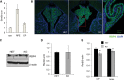
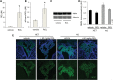
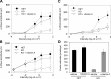
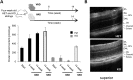
Vitamin A must be adequately distributed within the body to maintain the functions of retinoids in the periphery and chromophore production in the eyes. Blood transport of the lipophilic vitamin is mediated by the retinol-binding protein, RBP4. Biochemical evidence suggests that cellular uptake of vitamin A from RBP4 is facilitated by a membrane receptor. This receptor, identified as the Stimulated by retinoic acid gene 6 (Stra6) gene product, is highly expressed in epithelia that constitute blood-tissue barriers. Here we established a Stra6 knockout mouse model to analyze the metabolic basis of vitamin A homeostasis in peripheral tissues. These mice were viable when bred on diets replete in vitamin A, but evidenced markedly reduced levels of ocular retinoids. Ophthalmic imaging and histology revealed malformations in the choroid and retinal pigmented epithelium, early cone photoreceptor cell death, and reduced lengths of rod outer segments. Similar to the blood-retina barrier in the RPE, vitamin A transport through the blood-cerebrospinal fluid barrier in the brain's choroid plexus was impaired. Notably, treatment with pharmacological doses of vitamin A restored vitamin A transport across these barriers and rescued the vision of Stra6(-/-) mice. Furthermore, under conditions mimicking vitamin A excess and deficiency, our analyses revealed that STRA6-mediated vitamin A uptake is a regulated process mandatory for ocular vitamin A uptake when RBP4 constitutes the only transport mode in vitamin A deficiency. These findings identifying STRA6 as a bona fide vitamin A transporter have important implications for disease states associated with impaired blood vitamin A homeostasis.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Shah S.P., Taylor A.E., Sowden J.C., Ragge N.K., Russell-Eggitt I., Rahi J.S., Gilbert C.E. Anophthalmos, microphthalmos, and typical coloboma in the United Kingdom: a prospective study of incidence and risk. Invest. Ophthalmol. Vis. Sci. 2011;52:558–564. - PubMed
-
- Gerth-Kahlert C., Williamson K., Ansari M., Rainger J.K., Hingst V., Zimmermann T., Tech S., Guthoff R.F., van Heyningen V., Fitzpatrick D.R. Clinical and mutation analysis of 51 probands with anophthalmia and/or severe microphthalmia from a single center. Mol. Genet. Genomic Med. 2013;1:15–31. - PMC - PubMed
-
- Golzio C., Martinovic-Bouriel J., Thomas S., Mougou-Zrelli S., Grattagliano-Bessieres B., Bonniere M., Delahaye S., Munnich A., Encha-Razavi F., Lyonnet S., et al. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am. J. Hum. Genet. 2007;80:1179–1187. - PMC - PubMed
-
- Pasutto F., Sticht H., Hammersen G., Gillessen-Kaesbach G., Fitzpatrick D.R., Nurnberg G., Brasch F., Schirmer-Zimmermann H., Tolmie J.L., Chitayat D., et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 2007;80:550–560. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

