Involvement of the Wbp pathway in the biosynthesis of Porphyromonas gingivalis lipopolysaccharide with anionic polysaccharide
- PMID: 24852504
- PMCID: PMC4031482
- DOI: 10.1038/srep05056
Involvement of the Wbp pathway in the biosynthesis of Porphyromonas gingivalis lipopolysaccharide with anionic polysaccharide
Abstract
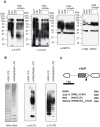
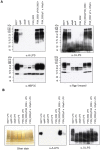
The periodontal pathogen Porphyromonas gingivalis has two different lipopolysaccharide (LPS) molecules, O-LPS and A-LPS. We have recently shown that P. gingivalis strain HG66 lacks A-LPS. Here, we found that introduction of a wild-type wbpB gene into strain HG66 restored formation of A-LPS. Sequencing of the wbpB gene from strain HG66 revealed the presence of a nonsense mutation in the gene. The wbpB gene product is a member of the Wbp pathway, which plays a role in the synthesis of UDP-ManNAc(3NAc)A in Pseudomonas aeruginosa; UDP-ManNAc(3NAc)A is sequentially synthesized by the WbpA, WbpB, WbpE, WbpD and WbpI proteins. We then determined the effect of the PGN_0002 gene, a wbpD homolog, on the biosynthesis of A-LPS. A PGN_0002-deficient mutant demonstrated an A-LPS biosynthesis deficiency. Taken together with previous studies, the present results suggest that the final product synthesized by the Wbp pathway is one of the sugar substrates necessary for the biosynthesis of A-LPS.
Figures




Similar articles
-
Biosynthesis of UDP-GlcNAc(3NAc)A by WbpB, WbpE, and WbpD: enzymes in the Wbp pathway responsible for O-antigen assembly in Pseudomonas aeruginosa PAO1.Biochemistry. 2009 Jun 16;48(23):5446-55. doi: 10.1021/bi900186u. Biochemistry. 2009. PMID: 19348502 Free PMC article.
-
Biosynthesis of a rare di-N-acetylated sugar in the lipopolysaccharides of both Pseudomonas aeruginosa and Bordetella pertussis occurs via an identical scheme despite different gene clusters.J Bacteriol. 2008 Sep;190(18):6060-9. doi: 10.1128/JB.00579-08. Epub 2008 Jul 11. J Bacteriol. 2008. PMID: 18621892 Free PMC article.
-
Characterization of WbpB, WbpE, and WbpD and reconstitution of a pathway for the biosynthesis of UDP-2,3-diacetamido-2,3-dideoxy-D-mannuronic acid in Pseudomonas aeruginosa.J Biol Chem. 2009 May 1;284(18):11854-62. doi: 10.1074/jbc.M808583200. Epub 2009 Mar 12. J Biol Chem. 2009. PMID: 19282284 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
-
Porphyromonas gingivalis: major periodontopathic pathogen overview.J Immunol Res. 2014;2014:476068. doi: 10.1155/2014/476068. Epub 2014 Mar 25. J Immunol Res. 2014. PMID: 24741603 Free PMC article. Review.
Cited by
-
PorA, a conserved C-terminal domain-containing protein, impacts the PorXY-SigP signaling of the type IX secretion system.Sci Rep. 2020 Dec 3;10(1):21109. doi: 10.1038/s41598-020-77987-y. Sci Rep. 2020. PMID: 33273542 Free PMC article.
-
Transcriptional modulation of Porphyromonas gingivalis biofilms on titanium-copper implant surfaces.Folia Microbiol (Praha). 2025 Feb 15. doi: 10.1007/s12223-025-01246-8. Online ahead of print. Folia Microbiol (Praha). 2025. PMID: 39955444
-
PorZ, an Essential Component of the Type IX Secretion System of Porphyromonas gingivalis, Delivers Anionic Lipopolysaccharide to the PorU Sortase for Transpeptidase Processing of T9SS Cargo Proteins.mBio. 2021 Feb 23;12(1):e02262-20. doi: 10.1128/mBio.02262-20. mBio. 2021. PMID: 33622730 Free PMC article.
-
Purification and characterisation of recombinant His-tagged RgpB gingipain from Porphymonas gingivalis.Biol Chem. 2015 Apr;396(4):377-84. doi: 10.1515/hsz-2014-0304. Biol Chem. 2015. PMID: 25720118 Free PMC article.
-
PGN_0297 is an essential component of the type IX secretion system (T9SS) in Porphyromonas gingivalis: Tn-seq analysis for exhaustive identification of T9SS-related genes.Microbiol Immunol. 2019 Jan;63(1):11-20. doi: 10.1111/1348-0421.12665. Microbiol Immunol. 2019. PMID: 30599082 Free PMC article.
References
-
- Okamoto K. et al. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J. Biol. Chem. 273, 21225–21231 (1998). - PubMed
-
- Shoji M. et al. Construction and characterization of a nonpigmented mutant of Porphyromonas gingivalis: cell surface polysaccharide as an anchorage for gingipains. Microbiology 148, 1183–1191 (2002). - PubMed
-
- Sato K. et al. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J. Biol. Chem. 280, 8668–8677 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

