Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies
- PMID: 24852950
- PMCID: PMC4171021
- DOI: 10.4161/mabs.28965
Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies
Abstract
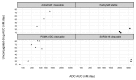
Although there are currently more than 30 antibody-drug conjugates (ADC) in clinical development for the treatment of blood cancers and solid tumors, comparison of their clinical pharmacokinetics (PK) is challenging because of the large number of, and differences between, the targets, ADC constructs, dosing regimens, and patient populations. In this review, we standardized the evaluation, using non-compartmental PK data reported at Cycle 1, i.e., following the first drug administration of what is usually a repeated-dose treatment, in monotherapy. We report ADC clinical PK properties, dosing regimen, determination of doses ranges and associated maximum tolerated doses. We also evaluated the effect of structural characteristics and target types (hematological vs. solid tumors) on PK. In addition, we discuss how integration of PK/pharmacodynamics approaches on top of classical dose escalation in first-in-human studies may improve dosing regimen determination for subsequent phases of clinical development.
Keywords: antibody-drug conjugates; dose selection; dosing regimen; maximum tolerated dose; oncology; pharmacokinetics; phase I.
Figures




References
-
- Gorovits B, Alley SC, Bilic S, Booth B, Kaur S, Oldfield P, Purushothama S, Rao C, Shord S, Siguenza P. Bioanalysis of antibody-drug conjugates: American Association of Pharmaceutical Scientists Antibody-Drug Conjugate Working Group position paper. Bioanalysis. 2013;5:997–1006. doi: 10.4155/bio.13.38. - DOI - PubMed
-
- Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R, Dumontet C, Morariu-Zamfir R, Lambert JM, Ozoux ML, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30:1121–31. doi: 10.1007/s10637-011-9670-0. - DOI - PubMed
-
- Lu D, Joshi A, Wang B, Olsen S, Yi JH, Krop IE, Burris HA, Girish S. An integrated multiple-analyte pharmacokinetic model to characterize trastuzumab emtansine (T-DM1) clearance pathways and to evaluate reduced pharmacokinetic sampling in patients with HER2-positive metastatic breast cancer. Clin Pharmacokinet. 2013;52:657–72. doi: 10.1007/s40262-013-0060-y. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
