Dual RNA-seq of parasite and host reveals gene expression dynamics during filarial worm-mosquito interactions
- PMID: 24853112
- PMCID: PMC4031193
- DOI: 10.1371/journal.pntd.0002905
Dual RNA-seq of parasite and host reveals gene expression dynamics during filarial worm-mosquito interactions
Abstract
Background: Parasite biology, by its very nature, cannot be understood without integrating it with that of the host, nor can the host response be adequately explained without considering the activity of the parasite. However, due to experimental limitations, molecular studies of parasite-host systems have been predominantly one-sided investigations focusing on either of the partners involved. Here, we conducted a dual RNA-seq time course analysis of filarial worm parasite and host mosquito to better understand the parasite processes underlying development in and interaction with the host tissue, from the establishment of infection to the development of infective-stage larva.
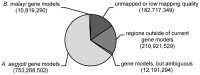
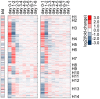
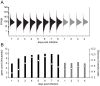
Methodology/principal findings: Using the Brugia malayi-Aedes aegypti system, we report parasite gene transcription dynamics, which exhibited a highly ordered developmental program consisting of a series of cyclical and state-transitioning temporal patterns. In addition, we contextualized these parasite data in relation to the concurrent dynamics of the host transcriptome. Comparative analyses using uninfected tissues and different host strains revealed the influence of parasite development on host gene transcription as well as the influence of the host environment on parasite gene transcription. We also critically evaluated the life-cycle transcriptome of B. malayi by comparing developmental stages in the mosquito relative to those in the mammalian host, providing insight into gene expression changes underpinning the mosquito-borne parasitic lifestyle of this heteroxenous parasite.
Conclusions/significance: The data presented herein provide the research community with information to design wet lab experiments and select candidates for future study to more fully dissect the whole set of molecular interactions of both organisms in this mosquito-filarial worm symbiotic relationship. Furthermore, characterization of the transcriptional program over the complete life cycle of the parasite, including stages within the mosquito, could help devise novel targets for control strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Global programme to eliminate lymphatic filariasis: progress report for 2012 (2013). Relevé Épidémiologique Hebd Sect Hygiène Secrétariat Société Nations Wkly Epidemiol Rec Health Sect Secr Leag Nations 88: : 389–399. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

