γδ T cells recognize the insulin B:9-23 peptide antigen when it is dimerized through thiol oxidation
- PMID: 24853397
- PMCID: PMC4091716
- DOI: 10.1016/j.molimm.2014.04.007
γδ T cells recognize the insulin B:9-23 peptide antigen when it is dimerized through thiol oxidation
Abstract
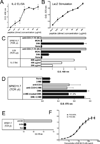
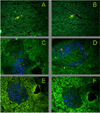
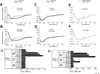
The insulin peptide B:9-23 is a natural antigen in the non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D). In addition to αβ T cells and B cells, γδ T cells recognize the peptide and infiltrate the pancreatic islets where the peptide is produced within β cells. The peptide contains a cysteine in position 19 (Cys19), which is required for the γδ but not the αβ T cell response, and a tyrosine in position 16 (Tyr16), which is required for both. A peptide-specific mAb, tested along with the T cells, required neither of the two amino acids to bind the B:9-23 peptide. We found that γδ T cells require Cys19 because they recognize the peptide antigen in an oxidized state, in which the Cys19 thiols of two peptide molecules form a disulfide bond, creating a soluble homo-dimer. In contrast, αβ T cells recognize the peptide antigen as a reduced monomer, in complex with the MHCII molecule I-A(g7). Unlike the unstructured monomeric B:9-23 peptide, the γδ-stimulatory homo-dimer adopts a distinct secondary structure in solution, which differs from the secondary structure of the corresponding portion of the native insulin molecule. Tyr16 is required for this adopted structure of the dimerized insulin peptide as well as for the γδ response to it. This observation is consistent with the notion that γδ T cell recognition depends on the secondary structure of the dimerized insulin B:9-23 antigen.
Keywords: Autoimmune diabetes; Autoreactivity; Gamma delta T cells; Insulin; Oxidation; T Cell Receptor.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23.J Autoimmun. 2010 Jun;34(4):478-84. doi: 10.1016/j.jaut.2009.12.008. Epub 2010 Jan 18. J Autoimmun. 2010. PMID: 20080388 Free PMC article.
-
Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes.J Autoimmun. 2019 Jan;96:50-58. doi: 10.1016/j.jaut.2018.08.004. Epub 2018 Aug 16. J Autoimmun. 2019. PMID: 30122420 Free PMC article.
-
Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse.Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16729-34. doi: 10.1073/pnas.1113954108. Epub 2011 Sep 26. Proc Natl Acad Sci U S A. 2011. PMID: 21949373 Free PMC article.
-
Structure of gammadelta T cell receptors and their recognition of non-peptide antigens.Mol Immunol. 2002 May;38(14):1051-61. doi: 10.1016/s0161-5890(02)00034-2. Mol Immunol. 2002. PMID: 11955597 Review.
-
Antigen recognition by human gammadelta T lymphocytes.Int Arch Allergy Immunol. 2000 May;122(1):1-7. doi: 10.1159/000024353. Int Arch Allergy Immunol. 2000. PMID: 10859464 Review.
Cited by
-
A Special Connection between γδ T Cells and Natural Antibodies?Arch Immunol Ther Exp (Warsz). 2016 Dec;64(6):455-462. doi: 10.1007/s00005-016-0403-0. Epub 2016 May 27. Arch Immunol Ther Exp (Warsz). 2016. PMID: 27235134 Free PMC article. Review.
-
The role of oxidative post-translational modifications in type 1 diabetes pathogenesis.Front Immunol. 2025 Feb 14;16:1537405. doi: 10.3389/fimmu.2025.1537405. eCollection 2025. Front Immunol. 2025. PMID: 40028329 Free PMC article. Review.
-
Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions.Cell Mol Immunol. 2020 Sep;17(9):914-924. doi: 10.1038/s41423-020-0503-y. Epub 2020 Jul 24. Cell Mol Immunol. 2020. PMID: 32709926 Free PMC article. Review.
-
The T cell antigen receptor: the Swiss army knife of the immune system.Clin Exp Immunol. 2015 Jul;181(1):1-18. doi: 10.1111/cei.12622. Epub 2015 May 14. Clin Exp Immunol. 2015. PMID: 25753381 Free PMC article. Review.
-
Autoantibody and T cell responses to oxidative post-translationally modified insulin neoantigenic peptides in type 1 diabetes.Diabetologia. 2023 Jan;66(1):132-146. doi: 10.1007/s00125-022-05812-4. Epub 2022 Oct 7. Diabetologia. 2023. PMID: 36207582 Free PMC article.
References
-
- Rast JP, Anderson M, Strong SJ, Luer C, Litman RT, Litman GW. α, β, γ and δ T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity. 1997;6:1–11. - PubMed
-
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10:467–478. - PubMed
-
- Mukasa A, Lahn M, Pflum EK, Born W, O'Brien RL. Evidence that the same gd T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

