Intestinal cell kinase, a protein associated with endocrine-cerebro-osteodysplasia syndrome, is a key regulator of cilia length and Hedgehog signaling
- PMID: 24853502
- PMCID: PMC4060650
- DOI: 10.1073/pnas.1323161111
Intestinal cell kinase, a protein associated with endocrine-cerebro-osteodysplasia syndrome, is a key regulator of cilia length and Hedgehog signaling
Abstract
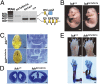
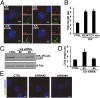
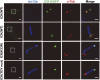
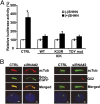
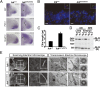
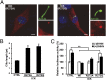
Endocrine-cerebro-osteodysplasia (ECO) syndrome is a recessive genetic disorder associated with multiple congenital defects in endocrine, cerebral, and skeletal systems that is caused by a missense mutation in the mitogen-activated protein kinase-like intestinal cell kinase (ICK) gene. In algae and invertebrates, ICK homologs are involved in flagellar formation and ciliogenesis, respectively. However, it is not clear whether this role of ICK is conserved in mammals and how a lack of functional ICK results in the characteristic phenotypes of human ECO syndrome. Here, we generated Ick knockout mice to elucidate the precise role of ICK in mammalian development and to examine the pathological mechanisms of ECO syndrome. Ick null mouse embryos displayed cleft palate, hydrocephalus, polydactyly, and delayed skeletal development, closely resembling ECO syndrome phenotypes. In cultured cells, down-regulation of Ick or overexpression of kinase-dead or ECO syndrome mutant ICK resulted in an elongation of primary cilia and abnormal Sonic hedgehog (Shh) signaling. Wild-type ICK proteins were generally localized in the proximal region of cilia near the basal bodies, whereas kinase-dead ICK mutant proteins accumulated in the distal part of bulged ciliary tips. Consistent with these observations in cultured cells, Ick knockout mouse embryos displayed elongated cilia and reduced Shh signaling during limb digit patterning. Taken together, these results indicate that ICK plays a crucial role in controlling ciliary length and that ciliary defects caused by a lack of functional ICK leads to abnormal Shh signaling, resulting in congenital disorders such as ECO syndrome.
Keywords: Gli2; LF4; MRK; Smoothened; ciliopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activation of sonic hedgehog signaling by a Smoothened agonist restores congenital defects in mouse models of endocrine-cerebro-osteodysplasia syndrome.EBioMedicine. 2019 Nov;49:305-317. doi: 10.1016/j.ebiom.2019.10.016. Epub 2019 Oct 26. EBioMedicine. 2019. PMID: 31662288 Free PMC article.
-
An essential role of intestinal cell kinase in lung development is linked to the perinatal lethality of human ECO syndrome.FEBS Lett. 2017 May;591(9):1247-1257. doi: 10.1002/1873-3468.12644. Epub 2017 Apr 21. FEBS Lett. 2017. PMID: 28380258 Free PMC article.
-
ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport.EMBO J. 2014 Jun 2;33(11):1227-42. doi: 10.1002/embj.201488175. Epub 2014 May 5. EMBO J. 2014. PMID: 24797473 Free PMC article.
-
The relationship between sonic Hedgehog signaling, cilia, and neural tube defects.Birth Defects Res A Clin Mol Teratol. 2010 Aug;88(8):633-52. doi: 10.1002/bdra.20686. Birth Defects Res A Clin Mol Teratol. 2010. PMID: 20544799 Free PMC article. Review.
-
Modeling ciliopathies: Primary cilia in development and disease.Curr Top Dev Biol. 2008;84:249-310. doi: 10.1016/S0070-2153(08)00605-4. Curr Top Dev Biol. 2008. PMID: 19186246 Review.
Cited by
-
Ciliopathy-Associated Protein Kinase ICK Requires Its Non-Catalytic Carboxyl-Terminal Domain for Regulation of Ciliogenesis.Cells. 2019 Jul 4;8(7):677. doi: 10.3390/cells8070677. Cells. 2019. PMID: 31277411 Free PMC article.
-
Multiscale modelling of relationships between protein classes and drug behavior across all diseases using the CANDO platform.Mini Rev Med Chem. 2015;15(8):705-17. doi: 10.2174/1389557515666150219145148. Mini Rev Med Chem. 2015. PMID: 25694071 Free PMC article.
-
LF4/MOK and a CDK-related kinase regulate the number and length of cilia in Tetrahymena.PLoS Genet. 2019 Jul 24;15(7):e1008099. doi: 10.1371/journal.pgen.1008099. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31339880 Free PMC article.
-
Ciliogenesis associated kinase 1: targets and functions in various organ systems.FEBS Lett. 2019 Nov;593(21):2990-3002. doi: 10.1002/1873-3468.13600. Epub 2019 Sep 20. FEBS Lett. 2019. PMID: 31506943 Free PMC article. Review.
-
The DYF-5 RCK and CDKL-1 CDKL5 kinases contribute differentially to shape distinct sensory neuron cilia morphologies in C. elegans.MicroPubl Biol. 2022 Aug 4;2022:10.17912/micropub.biology.000619. doi: 10.17912/micropub.biology.000619. eCollection 2022. MicroPubl Biol. 2022. PMID: 35996689 Free PMC article.
References
-
- Togawa K, Yan YX, Inomoto T, Slaugenhaupt S, Rustgi AK. Intestinal cell kinase (ICK) localizes to the crypt region and requires a dual phosphorylation site found in map kinases. J Cell Physiol. 2000;183(1):129–139. - PubMed
-
- Miyata Y, Nishida E. Distantly related cousins of MAP kinase: Biochemical properties and possible physiological functions. Biochem Biophys Res Commun. 1999;266(2):291–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

