Voltage and calcium dynamics both underlie cellular alternans in cardiac myocytes
- PMID: 24853751
- PMCID: PMC4052344
- DOI: 10.1016/j.bpj.2014.03.048
Voltage and calcium dynamics both underlie cellular alternans in cardiac myocytes
Abstract
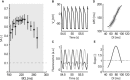
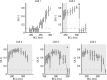
Cardiac alternans, a putative trigger event for cardiac reentry, is a beat-to-beat alternation in membrane potential and calcium transient. Alternans was originally attributed to instabilities in transmembrane ion channel dynamics (i.e., the voltage mechanism). As of this writing, the predominant view is that instabilities in subcellular calcium handling are the main underlying mechanism. That being said, because the voltage and calcium systems are bidirectionally coupled, theoretical studies have suggested that both mechanisms can contribute. To date, to our knowledge, no experimental evidence of such a dual role within the same cell has been reported. Here, a combined electrophysiological and calcium imaging approach was developed and used to illuminate the contributions of voltage and calcium dynamics to alternans. An experimentally feasible protocol, quantification of subcellular calcium alternans and restitution slope during cycle-length ramping alternans control, was designed and validated. This approach allows simultaneous illumination of the contributions of voltage and calcium-driven instability to total cellular instability as a function of cycle-length. Application of this protocol in in vitro guinea-pig left-ventricular myocytes demonstrated that both voltage- and calcium-driven instabilities underlie alternans, and that the relative contributions of the two systems change as a function of pacing rate.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Pastore J.M., Girouard S.D., Rosenbaum D.S. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. - PubMed
-
- Narayan S.M. T-wave alternans and the susceptibility to ventricular arrhythmias. J. Am. Coll. Cardiol. 2006;47:269–281. - PubMed
-
- Rosenbaum D.S., Jackson L.E., Cohen R.J. Electrical alternans and vulnerability to ventricular arrhythmias. N. Engl. J. Med. 1994;330:235–241. - PubMed
-
- Fox J.J., McHarg J.L., Gilmour R.F., Jr. Ionic mechanism of electrical alternans. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H516–H530. - PubMed
-
- Koller M.L., Riccio M.L., Gilmour R.F., Jr. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am. J. Physiol. 1998;275:H1635–H1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

