Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain
- PMID: 24853772
- PMCID: PMC4207343
- DOI: 10.1038/npp.2014.117
Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain
Abstract
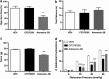
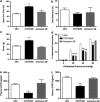
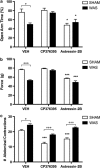
Corticotropin-releasing factor (CRF)-mediated mechanisms in the bed nucleus of the stria terminalis (BNST) have a pivotal role in stress-induced anxiety and hyperalgesia. Although CRF is known to activate two receptor subtypes, CRF1 and CRF2, attempts to delineate the specific role of each subtype in modulating anxiety and nociception have been inconsistent. Here we test the hypothesis that CRF1 and CRF2 receptor activation in the anteriolateral BNST (BNSTAL) facilitates divergent mechanisms modulating comorbid anxiety and hyperalgesia. Microinfusions of the specific antagonists CP376395 and Astressin2B into the BNSTAL were used to investigate CRF1 and CRF2 receptor functions, respectively. We found that CRF1 and CRF2 receptors in the BNSTAL had opposing effects on exploratory behavior in the elevated plus-maze, somatic mechanical threshold, and the autonomic and endocrine response to stress. However, CRF1 or CRF2 receptor antagonism in the BNSTAL revealed complementary roles in facilitating the acoustic startle and visceromotor reflexes. Our results suggest that the net effect of CRF1 and CRF2 receptor activation in the BNSTAL is pathway-dependent and provides important insight into the CRF receptor-associated circuitry that likely underpins stress-induced pathologies.
Figures



References
-
- Allen C, Kendall JW. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967;80:926–930. - PubMed
-
- Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, et al. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. - PubMed
-
- Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. - PubMed
-
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

