Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia
- PMID: 24853871
- PMCID: PMC4031164
- DOI: 10.1371/journal.pntd.0002871
Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia
Abstract
Background: Pentavalent antimonials have been the first line treatment for dermal leishmaniasis in Colombia for over 30 years. Miltefosine is administered as second line treatment since 2005. The susceptibility of circulating populations of Leishmania to these drugs is unknown despite clinical evidence supporting the emergence of resistance.
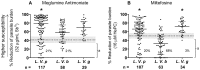
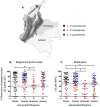
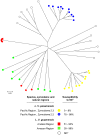
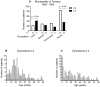
Methodology/principal findings: In vitro susceptibility was determined for intracellular amastigotes of 245 clinical strains of the most prevalent Leishmania Viannia species in Colombia to miltefosine (HePC) and/or meglumine antimoniate (Sb(V)); 163, (80%) were evaluated for both drugs. Additionally, susceptibility to Sb(V) was examined in two cohorts of 85 L. V. panamensis strains isolated between 1980-1989 and 2000-2009 in the municipality of Tumaco. Susceptibility to each drug differed among strains of the same species and between species. Whereas 68% of L. V. braziliensis strains presented in vitro resistance to HePC, 69% were sensitive to Sb(V). Resistance to HePC and Sb(V) occurred respectively, in 20% y 21% of L. panamensis strains. Only 3% of L. V. guyanensis were resistant to HePC, and none to Sb(V). Drug susceptibility differed between geographic regions and time periods. Subpopulations having disparate susceptibility to Sb(V) were discerned among L. V. panamensis strains isolated during 1980-1990 in Tumaco where resistant strains belonged to zymodeme 2.3, and sensitive strains to zymodeme 2.2.
Conclusions/significance: Large scale evaluation of clinical strains of Leishmania Viannia species demonstrated species, population, geographic, and epidemiologic differences in susceptibility to meglumine antimoniate and miltefosine, and provided baseline information for monitoring susceptibility to these drugs. Sensitive and resistant clinical strains within each species, and zymodeme as a proxy marker of antimony susceptibility for L. V. panamensis, will be useful in deciphering factors involved in susceptibility and the distribution of sensitive and resistant populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Ait-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D (2011) Leishmania antimony resistance: what we know what we can learn from the field. Parasitol Res 109: 1225–1232. - PubMed
-
- Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, et al. (2008) Treatment of New World cutaneous leishmaniasis—a systematic review with a meta-analysis. Int J Dermatol 47: 109–124. - PubMed
-
- Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, et al. (2004) Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis 38: 1266–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

