Pericyte dynamics during angiogenesis: new insights from new identities
- PMID: 24853910
- PMCID: PMC4149862
- DOI: 10.1159/000362276
Pericyte dynamics during angiogenesis: new insights from new identities
Abstract
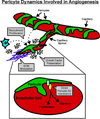
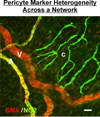
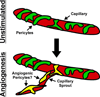
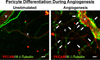
Therapies aimed at manipulating the microcirculation require the ability to control angiogenesis, defined as the sprouting of new capillaries from existing vessels. Blocking angiogenesis would be beneficial in many pathologies (e.g. cancer, retinopathies and rheumatoid arthritis). In others (e.g. myocardial infarction, stroke and hypertension), promoting angiogenesis would be desirable. We know that vascular pericytes elongate around endothelial cells (ECs) and are functionally associated with regulating vessel stabilization, vessel diameter and EC proliferation. During angiogenesis, bidirectional pericyte-EC signaling is critical for capillary sprout formation. Observations of pericytes leading capillary sprouts also implicate their role in EC guidance. As such, pericytes have recently emerged as a therapeutic target to promote or inhibit angiogenesis. Advancing our basic understanding of pericytes and developing pericyte-related therapies are challenged, like in many other fields, by questions regarding cell identity. This review article discusses what we know about pericyte phenotypes and the opportunity to advance our understanding by defining the specific pericyte cell populations involved in capillary sprouting.
© 2014 S. Karger AG, Basel.
Figures
Similar articles
-
Weibel-Palade Bodies Orchestrate Pericytes During Angiogenesis.Arterioscler Thromb Vasc Biol. 2019 Sep;39(9):1843-1858. doi: 10.1161/ATVBAHA.119.313021. Epub 2019 Jul 18. Arterioscler Thromb Vasc Biol. 2019. PMID: 31315435
-
Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche.Histol Histopathol. 2009 Jul;24(7):909-69. doi: 10.14670/HH-24.909. Histol Histopathol. 2009. PMID: 19475537 Review.
-
Identification of class III β-tubulin as a marker of angiogenic perivascular cells.Microvasc Res. 2012 Mar;83(2):257-62. doi: 10.1016/j.mvr.2011.09.003. Epub 2011 Sep 17. Microvasc Res. 2012. PMID: 21958528
-
Targeting pericytes for angiogenic therapies.Microcirculation. 2014 May;21(4):345-57. doi: 10.1111/micc.12107. Microcirculation. 2014. PMID: 24267154 Free PMC article. Review.
-
Understanding angiogenesis during aging: opportunities for discoveries and new models.J Appl Physiol (1985). 2018 Dec 1;125(6):1843-1850. doi: 10.1152/japplphysiol.00112.2018. Epub 2018 Apr 12. J Appl Physiol (1985). 2018. PMID: 29648521 Free PMC article. Review.
Cited by
-
Pericytes as the Orchestrators of Vasculature and Adipogenesis.Genes (Basel). 2024 Jan 19;15(1):126. doi: 10.3390/genes15010126. Genes (Basel). 2024. PMID: 38275607 Free PMC article. Review.
-
Microvascular dysfunction and kidney disease: Challenges and opportunities?Microcirculation. 2021 Apr;28(3):e12661. doi: 10.1111/micc.12661. Epub 2020 Oct 28. Microcirculation. 2021. PMID: 33025626 Free PMC article. Review.
-
Chondroitin Sulfate Proteoglycan 4 as a Marker for Aggressive Squamous Cell Carcinoma.Cancers (Basel). 2022 Nov 13;14(22):5564. doi: 10.3390/cancers14225564. Cancers (Basel). 2022. PMID: 36428658 Free PMC article. Review.
-
Combined effects of pericytes in the tumor microenvironment.Stem Cells Int. 2015;2015:868475. doi: 10.1155/2015/868475. Epub 2015 Apr 27. Stem Cells Int. 2015. PMID: 26000022 Free PMC article. Review.
-
Cocaine Mediated Neuroinflammation: Role of Dysregulated Autophagy in Pericytes.Mol Neurobiol. 2019 May;56(5):3576-3590. doi: 10.1007/s12035-018-1325-0. Epub 2018 Aug 27. Mol Neurobiol. 2019. PMID: 30151726 Free PMC article.
References
-
- Zimmermann KW. Der feinere Bau der Blutkapillaren. Z Anat. 1923;68:29–109.
-
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. - PubMed
-
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. - PubMed
-
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFRbeta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. - PubMed
-
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

