Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway
- PMID: 24854034
- PMCID: PMC4031079
- DOI: 10.1371/journal.pntd.0002872
Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway
Abstract
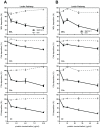
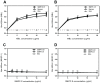
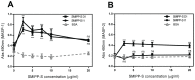
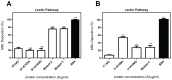
Scabies is an infectious skin disease caused by the mite Sarcoptes scabiei and has been classified as one of the six most prevalent epidermal parasitic skin diseases infecting populations living in poverty by the World Health Organisation. The role of the complement system, a pivotal component of human innate immunity, as an important defence against invading pathogens has been well documented and many parasites have an arsenal of anti-complement defences. We previously reported on a family of scabies mite proteolytically inactive serine protease paralogues (SMIPP-Ss) thought to be implicated in host defence evasion. We have since shown that two family members, SMIPP-S D1 and I1 have the ability to bind the human complement components C1q, mannose binding lectin (MBL) and properdin and are capable of inhibiting all three human complement pathways. This investigation focused on inhibition of the lectin pathway of complement activation as it is likely to be the primary pathway affecting scabies mites. Activation of the lectin pathway relies on the activation of MBL, and as SMIPP-S D1 and I1 have previously been shown to bind MBL, the nature of this interaction was examined using binding and mutagenesis studies. SMIPP-S D1 bound MBL in complex with MBL-associated serine proteases (MASPs) and released the MASP-2 enzyme from the complex. SMIPP-S I1 was also able to bind MBL in complex with MASPs, but MASP-1 and MASP-2 remained in the complex. Despite these differences in mechanism, both molecules inhibited activation of complement components downstream of MBL. Mutagenesis studies revealed that both SMIPP-Ss used an alternative site of the molecule from the residual active site region to inhibit the lectin pathway. We propose that SMIPP-Ss are potent lectin pathway inhibitors and that this mechanism represents an important tool in the immune evasion repertoire of the parasitic mite and a potential target for therapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bergstrom FC, Reynolds S, Johnstone M, Pike RN, Buckle AM, et al. (2009) Scabies mite inactivated serine protease paralogs inhibit the human complement system. J Immunol 182: 7809–7817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

